Azithromycin for treating uncomplicated malaria
- PMID: 21328286
- PMCID: PMC6532599
- DOI: 10.1002/14651858.CD006688.pub2
Azithromycin for treating uncomplicated malaria
Abstract
Background: To prevent the development of drug resistance, the World Health Organization (WHO) recommends treating malaria with combination therapy. Azithromycin, an antibiotic with antimalarial properties, may be a useful additional option for antimalarial therapy.
Objectives: To compare the use of azithromycin alone or in combination with other antimalarial drugs with the use of alternative antimalarial drugs for treating uncomplicated malaria caused by Plasmodium falciparum or Plasmodium vivax.
Search strategy: We searched the Cochrane Infectious Diseases Group Specialized Register (August 2010); CENTRAL (The Cochrane Library Issue 3, 2010); MEDLINE (1966 to August 2010); EMBASE (1974 to August 2010); LILACS (August 2010); the metaRegister of Controlled Trials (mRCT, August 2010); conference proceedings; and reference lists. We also contacted researchers and a pharmaceutical company.
Selection criteria: Randomized controlled trials comparing azithromycin, either alone or combined with another antimalarial drug, with another antimalarial drug used alone or combined with another antimalarial drug, or with azithromycin combined with another antimalarial drug if different combinations or doses of azithromycin were used. The primary outcome was treatment failure by day 28, defined as parasitological or clinical evidence of treatment failure between the start of treatment and day 28. Secondary outcomes included treatment failure by day 28 corrected for new infections confirmed by polymerase chain reaction (PCR), fever and parasite clearance time, and adverse events.
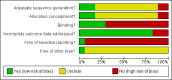
Data collection and analysis: Two people independently applied the inclusion criteria, extracted data and assessed methodological quality. We used risk ratio (RR) and 95% confidence intervals (CI).
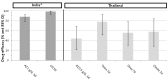
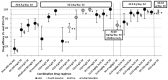
Main results: Fifteen trials met the inclusion criteria (2284 participants, 69% males, 16% children). They were conducted in disparate malaria endemic areas, with the earlier studies conducted in Thailand (five) and India (two), and the more recent studies (eight) spread across three continents (South America, Africa, Asia). The 15 studies involved 41 treatment arms, 12 different drugs, and 28 different treatment regimens. Two studies examined P. vivax.Three-day azithromycin (AZ) monotherapy did not perform well for P. vivax or P. falciparum (Thailand: P. vivax failure rate 0.5 g daily, 56%, 95% CI 31 to 78. India: P. vivax failure rate 1 g daily,12%, 95% CI 7 to 21; P. falciparum failure rate 1 g daily, 64%, 95% CI 36 to 86.) A 1 g azithromycin and 0.6 g chloroquine combination daily for three days for uncomplicated P. falciparum infections was associated with increased treatment failure in India and Indonesia compared with the combination of sulphadoxine-pyrimethamine and chloroquine (pooled RR 2.66, 95% CI 1.25 to 5.67), and compared with the combination atovaquone-proguanil in a multicentre trial in Columbia and Surinam (RR 24.72, 95% CI 6.16 to 99.20). No increased risk of treatment failure was seen in two studies in Africa with mefloquine as the comparator drug (pooled RR 2.02, 95% CI 0.51 to 7.96, P = 0.3); the pooled RR for PCR-corrected data for the combination versus mefloquine was 1.01, 95% CI 0.18 to 5.84 (P = 1.0). An increased treatment failure risk was seen when comparing azithromycin in a dose of 1.2 to 1.5 mg in combination with artesunate (200 mg per day for three days) with artemether-lumefantrine (pooled RR 3.08, 95% CI 2.09 to 4.55; PCR-corrected pooled RR 3.63, 95% CI 2.02 to 6.52).Serious adverse events and treatment discontinuation were similar across treatment arms. More adverse events were reported when comparing the 1 g azithromycin/ 0.6 g chloroquine combination with mefloquine (pooled RR 1.20, 95% CI 1.06 to 1.36) or atovaquone-proguanil (RR 1.41, 95% CI 1.09 to1.83).
Authors' conclusions: Currently, there is no evidence for the superiority or equivalence of azithromycin monotherapy or combination therapy for the treatment of P. falciparum or P. vivax compared with other antimalarials or with the current first-line antimalarial combinations. The available evidence suggests that azithromycin is a weak antimalarial with some appealing safety characteristics. Unless the ongoing dose, formulation and product optimisation process results in a universally efficacious product, or a specific niche application is identified that is complementary to the current scala of more efficacious antimalarial combinations, azithromycin's future for the treatment of malaria does not look promising.
Conflict of interest statement
None known.
Figures










Update of
- doi: 10.1002/14651858.CD006688
Similar articles
-
Artemisinin-based combination therapy for treating uncomplicated Plasmodium vivax malaria.Cochrane Database Syst Rev. 2013 Oct 25;2013(10):CD008492. doi: 10.1002/14651858.CD008492.pub3. Cochrane Database Syst Rev. 2013. PMID: 24163021 Free PMC article.
-
Artemisinin-based combination therapy for treating uncomplicated malaria.Cochrane Database Syst Rev. 2009 Jul 8;2009(3):CD007483. doi: 10.1002/14651858.CD007483.pub2. Cochrane Database Syst Rev. 2009. PMID: 19588433 Free PMC article.
-
Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2014 Jan 20;2014(1):CD010927. doi: 10.1002/14651858.CD010927. Cochrane Database Syst Rev. 2014. PMID: 24443033 Free PMC article.
-
Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2014 Mar 4;(3):CD006404. doi: 10.1002/14651858.CD006404.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Jan 08;1:CD006404. doi: 10.1002/14651858.CD006404.pub3. PMID: 24596021 Free PMC article. Updated.
-
Primaquine for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008152. doi: 10.1002/14651858.CD008152.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. PMID: 22972117 Updated.
Cited by
-
Optimal seasonal timing of oral azithromycin for malaria.Am J Trop Med Hyg. 2014 Nov;91(5):936-942. doi: 10.4269/ajtmh.13-0474. Epub 2014 Sep 15. Am J Trop Med Hyg. 2014. PMID: 25223942 Free PMC article.
-
A novel class of fast-acting antimalarial agents: Substituted 15-membered azalides.Br J Pharmacol. 2021 Jan;178(2):363-377. doi: 10.1111/bph.15292. Epub 2020 Dec 16. Br J Pharmacol. 2021. PMID: 33085774 Free PMC article.
-
Targeting malaria parasites with novel derivatives of azithromycin.Front Cell Infect Microbiol. 2022 Nov 30;12:1063407. doi: 10.3389/fcimb.2022.1063407. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36530422 Free PMC article.
-
Treatment of adults with acute uncomplicated malaria with azithromycin and chloroquine in India, Colombia, and Suriname.Res Rep Trop Med. 2017 Oct 13;8:85-104. doi: 10.2147/RRTM.S129741. eCollection 2017. Res Rep Trop Med. 2017. PMID: 30050349 Free PMC article.
-
The impact on malaria of biannual treatment with azithromycin in children age less than 5 years: a prospective study.Malar J. 2019 Aug 23;18(1):284. doi: 10.1186/s12936-019-2914-8. Malar J. 2019. PMID: 31443654 Free PMC article. Clinical Trial.
References
References to studies included in this review
-
- Dunne MW, Singh N, Shukla M, Valecha N, Bhattacharyya PC, Patel K, et al. A double‐blind, randomized study of azithromycin compared to chloroquine for the treatment of Plasmodium vivax malaria in India. American Journal of Tropical Medicine and Hygiene 2005;73(6):1108‐11. - PubMed
-
- Dunne MW, Singh N, Shukla M, Valecha N, Bhattacharyya PC, Dev V, et al. A multicenter study of azithromycin, alone and in combination with chloroquine, for the treatment of acute uncomplicated Plasmodium falciparum malaria in India. Journal of Infectious Diseases 2005;191(10):1582‐8. - PubMed
-
- Krudsood S, Silachamroon U, Wilairatana P, Singhasivanon P, Phumratanaprapin W, Chalermrut K, et al. A randomized clinical trial of combinations of artesunate and azithromycin for treatment of uncomplicated Plasmodium falciparum malaria in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 2000;31(4):801‐7. - PubMed
-
- Krudsood S, Buchachart K, Chalermut K, Charusabha C, Treepasertsuk S, Haoharn O, et al. A comparative clinical trial of combinations of dihydroartemisinin plus azithromycin and dihydroartemisinin plus mefloquine for treatment of multidrug resistant falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 2002;33(3):525‐31. - PubMed
-
- Miller RS, Wongsrichanalai C, Buathong N, McDaniel P, Walsh DS, Knirsch C, et al. Effective treatment of uncomplicated Plasmodium falciparum malaria with azithromycin‐quinine combinations: a randomized, dose ranging study. American Journal of Tropical Medicine and Hygiene 2006;74(3):401‐6. - PubMed
References to ongoing studies
-
- National Institute of Allergy and Infectious Diseases. Chloroquine alone or in combination for malaria in children in Malawi. http://www.clinicaltrials.gov/ct/show/NCT00379821.2006.
-
- Pfizer Inc. Azithromycin plus chloroquine versus artemether‐lumefantrine for the treatment of uncomplicated P. falciparum malaria in children in Africa. http://www.clinicaltrials.gov/ct/show/NCT00677833.2008. - PMC - PubMed
Additional references
-
- Adair CD, Gunter M, Stovall TG, McElroy G, Veille JC, Ernest JM. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin. Obstetrics and Gynecology 1998;91(2):165‐8. - PubMed
-
- Andersen SL, Ager A, McGreevy P, Schuster BG, Wesche D, Kuschner R, et al. Activity of azithromycin as a blood schizonticide against rodent and human plasmodium in vivo. American Journal of Tropical Medicine and Hygiene 1995;52(2):159‐61. - PubMed
-
- Andersen SL, Oloo AJ, Gordon DM, Ragama OB, Aleman GM, Bermand JD, et al. Successful double‐blinded, randomized, placebo‐controlled field trial of azithromycin and doxycycline as prophylaxis for malaria in Western Kenya. Clinical Infectious Diseases 1998;26:146‐50. - PubMed
-
- Barnes KI, Watkins WM, White NJ. Antimalarial dosing regimens and drug resistance. Trends in Parasitology 2008;24(3):127‐134. - PubMed
-
- Biswas S. In‐vitro antimalarial activity of azithromycin against chloroquine sensitive and chloroquine resistant Plasmodium falciparum. Journal of Postgraduate Medicine 2001;47(4):240‐3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

