Pharmacologic treatment of depression in multiple sclerosis
- PMID: 21328292
- PMCID: PMC12070153
- DOI: 10.1002/14651858.CD007295.pub2
Pharmacologic treatment of depression in multiple sclerosis
Abstract
Background: Depression is a common problem in patients with multiple sclerosis (MS). It is unclear which pharmacologic treatment is the most effective and the least harmful.
Objectives: To investigate the efficacy and tolerability of pharmacologic treatments for depression in patients with MS.
Search strategy: We searched the Cochrane Multiple Sclerosis Group's Trials Register (June 2010), reference lists of relevant articles and conference proceedings. Regulatory agencies were used as additional sources of information on adverse effects.
Selection criteria: Adequately and quasi-randomized controlled blinded or unblinded trials in children and adults with MS.
Experimental intervention: pharmacologic treatments for depression without restrictions regarding dose, route of administration, frequency, or duration. Control intervention: placebo treatment or no treatment.
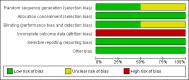
Data collection and analysis: Two teams of reviewers independently assessed trial quality and extracted data. We contacted study authors for additional information. We collected adverse effects from the trials.Information about study population, type of intervention, outcome measures, and study design were extracted from the selected studies. Trial quality was evaluated with the criteria: randomization, allocation concealment, blinding, handling of incomplete outcome data, freedom from selective reporting and freedom from other bias.The impact of missing data on the study results was explored with sensitivity analyses comparing the results from the analyses of study completers with those from best- and worst-case scenarios.
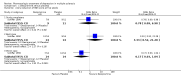
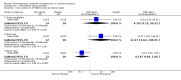
Main results: Two trials (70 participants) were included. One trial (28 participants) compared treatment with desipramine for five weeks to placebo. The other trial (42 participants) compared treatment with paroxetine for twelve weeks to placebo. Both trials had a significant number of patients lost to follow-up or with missing outcome measurements.There was a trend towards efficacy of both treatments compared to placebo, but this difference was not statistically significant except for one outcome. Confidence intervals were wide in all analyses and our sensitivity analysis showed that the missing data may have had an important effect in both trials, with large differences between best-case and worst-case scenarios for all assessed outcomes.Both treatments were associated with adverse effects, with significantly more patients treated with paroxetine suffering from nausea or headache. Given the difference in trial duration and type of drug, we decided not to perform a meta-analysis.
Authors' conclusions: Both desipramine and paroxetine show a trend towards efficacy in depression in MS the short term, but both treatments were associated with adverse effects, with significantly more patients treated with paroxetine suffering from nausea or headache. Further clinical research on the treatment of depression in MS is clearly needed. Future trials should address the efficacy and tolerability in the long term and compare antidepressant treatments head-to-head.
Conflict of interest statement
We declare we have no conflicts of interest.
Figures



























Update of
References
References to studies included in this review
Ehde 2008 {published and unpublished data}
-
- Ehde DM, Kraft GH, Chwastiak L, Sullivan MD, Gibbons LE, Bombardier CH, et al. Efficacy of paroxetine in treating major depressive disorder in persons with multiple sclerosis. General Hospital Psychiatry 2008;30:40‐8. - PubMed
Schiffer 1990 {published data only}
-
- Schiffer RB, Wineman NM. Antidepressant pharmacotherapy of depression associated with multiple sclerosis. American Journal of Psychiatry 1990;147:1493‐97. - PubMed
Additional references
Bech 2001
-
- Bech P, Rasmussen NA, Olsen LR, Noerholm V, Abildgaard W. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. Journal of Affective Disorders 2001;66(2‐3):159‐64. - PubMed
Beck 1961
-
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;12(1):561‐71. - PubMed
Derogatis 1973
-
- Derogatis LR, Lipman RS, Covi L. SCL‐90: an outpatient psychiatric rating scale‐‐preliminary report. Psychopharmacology Bulletin 1973;9(1):13‐28. - PubMed
Diener 1985
-
- Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. Journal of Personality Assessment 1985;49:71‐5. - PubMed
Endicott 1979
-
- Endicott J, Spitzer RL. Use of the Research Diagnostic Criteria and the Schedule for Affective Disorders and Schizophrenia to study affective disorders. American Journal of Psychiatry 1979;136:52‐6. - PubMed
First 1996
-
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM‐IV Axis I Disorders, Clinician Version (SCID‐CV). American Psychiatric Press, Inc., 1996.
Fischer 1999
-
- Fischer JS, Rocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in MS. Multiple Sclerosis 1999;5:251‐9. - PubMed
Hamilton 1960
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, Ltd., 2008.
McDonald 2001
-
- McDonald WI, Compston A, Edan G. Recommended diagnostic criteria for MS: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50:121‐7. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale, designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐92. - PubMed
Patten 2005
-
- Patten SB, Beck CA, Williams JVA, Barbui C, Metz LM. Major depression in multiple sclerosis: a population‐based perspective. Neurology 2003;61(11):1524‐7. - PubMed
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for Multiple Sclerosis: guidelines for research proposals. Annals of Neurology 1983;13:227‐31. - PubMed
Radloff 1977
-
- Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Applied Psychological Measures 1977;1:385‐401.
Rush 1986
-
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger JE, Burns CT. The Inventory of Depressive Symptomatology (IDS): Preliminary findings. Psychiatry Research 1986;18:65‐87. - PubMed
Siegert 2005
Sullivan 1990
-
- Sullivan JJ, Egdley K, Dehoux E. A survey of multiple sclerosis: Part 1. Perceived cognitive problems and compensatory strategy use. Canadian Journal of Rehabilitation 1990;4:99‐105.
Ware 1992
-
- Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey. Medical Care 1992;30:473‐83. - PubMed
Zigmond 1983
-
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. - PubMed
Zung 1965
-
- Zung WWK. A self‐rating depression scale. Archives of General Psychiatry 1965;12(1):63‐70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

