Stable isotope-resolved metabolomics (SIRM) in cancer research with clinical application to nonsmall cell lung cancer
- PMID: 21329461
- PMCID: PMC3125551
- DOI: 10.1089/omi.2010.0088
Stable isotope-resolved metabolomics (SIRM) in cancer research with clinical application to nonsmall cell lung cancer
Abstract
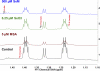
Metabolomics provides a readout of the state of metabolism in cells or tissue and their responses to external perturbations. For this reason, the approach has great potential in clinical diagnostics. Clinical metabolomics using stable isotope resolved metabolomics (SIRM) for pathway tracing represents an important new approach to obtaining metabolic parameters in human cancer subjects in situ. Here we provide an overview of the technology development of labeling from cells in culture and mouse models. The high throughput analytical methods NMR and mass spectrometry, especially Fourier transform ion cyclotron resonance, for analyzing the resulting metabolite isotopomers and isotopologues are described with examples of applications in cancer biology. Special technical considerations for clinical applications of metabolomics using stable isotope tracers are described. The whole process from concept to analysis will be exemplified by our on-going study of nonsmall cell lung cancer (NSCLC) metabolomics. This powerful new approach has already provided important new insights into metabolic adaptations in lung cancer cells, including the upregulation of anaplerosis via pyruvate carboxylation in NSCLC.
Figures
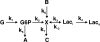






References
-
- Cancer Facts and Figures—2008. American Cancer Society; Boston, MA: ACS. (2008) pp. 1–72.
-
- Boyer M.J. Tannock I.F. Cellular and molecular basis of chemotherapy. In: Tannock I.F, editor; Hill R.P., editor. The Basic Science of Oncology. McGraw Hill; New York: 1998. pp. 350–369.
-
- Editorial. Early warnings. Nature. 2009;458:679–679. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

