Transcriptional activators enhance polyadenylation of mRNA precursors
- PMID: 21329879
- PMCID: PMC3060669
- DOI: 10.1016/j.molcel.2011.01.022
Transcriptional activators enhance polyadenylation of mRNA precursors
Abstract
Polyadenylation of mRNA precursors is frequently coupled to transcription by RNA polymerase II. Although this coupling is known to involve interactions with the C-terminal domain of the RNA polymerase II largest subunit, the possible role of other factors is not known. Here we show that a prototypical transcriptional activator, GAL4-VP16, stimulates transcription-coupled polyadenylation in vitro. In the absence of GAL4-VP16, specifically initiated transcripts accumulated but little polyadenylation was observed, while in its presence polyadenylation was strongly enhanced. We further show that this stimulation requires the transcription elongation-associated PAF complex (PAF1c), as PAF1c depletion blocked GAL4-VP16-stimulated polyadenylation. Furthermore, knockdown of PAF subunits by siRNA resulted in decreased 3' cleavage, and nuclear export, of mRNA in vivo. Finally, we show that GAL4-VP16 interacts directly with PAF1c and recruits it to DNA templates. Our results indicate that a transcription activator can stimulate transcription-coupled 3' processing and does so via interaction with PAF1c.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
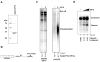


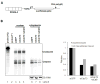



Comment in
-
Gene expression: Transcriptional activators do double-duty.Nat Rev Mol Cell Biol. 2011 Apr;12(4):205. doi: 10.1038/nrm3087. Epub 2011 Mar 16. Nat Rev Mol Cell Biol. 2011. PMID: 21407238 No abstract available.
References
-
- Bentley D. The mRNA assembly line: transcription and processing machines in the same factory. Curr Opin Cell Biol. 2002;14:336–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

