Potential of integrated [18F] fluorodeoxyglucose positron-emission tomography/CT in identifying vulnerable carotid plaques
- PMID: 21330389
- PMCID: PMC7965558
- DOI: 10.3174/ajnr.A2381
Potential of integrated [18F] fluorodeoxyglucose positron-emission tomography/CT in identifying vulnerable carotid plaques
Abstract
Background and purpose: There is a need for improved risk stratification of patients with TIA/stroke and carotid atherosclerosis. The purpose of this study was to prospectively investigate the potential of integrated (18)F-FDG PET/MDCT in identifying vulnerable carotid plaques.
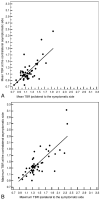
Materials and methods: Fifty patients with TIA/stroke with an ipsilateral carotid plaque causing <70% stenosis and a plaque on the contralateral asymptomatic side underwent integrated (18)F-FDG PET/MDCT within 36.1 ± 20.0 days (range, 9-95 days) of the last symptoms. Carotid plaque (18)F-FDG uptake was measured as both the mean and maximum blood-normalized SUV, known as the TBR. Using MDCT, we assessed volumes of vessel wall and individual plaque components.
Results: Mean TBR was only significantly larger in the ipsilateral plaques of patients who were imaged within 38 days (1.24 ± 0.04 [SE] versus 1.17 ± 0.05, P = .014). This also accounted for maximum TBR (1.53 ± 0.06 versus 1.42 ± 0.06, P = .015). MDCT-assessed vessel wall and LRNC volumes were larger in ipsilateral plaques of all patients (982.3 ± 121.3 versus 811.3 ± 106.6 mm(3), P = .016; 164.7 ± 26.1 versus 134.3 ± 35.2 mm(3), P = .026, respectively).
Conclusions: In the present study, (18)F-FDG PET only detected significant differences between ipsilateral and contralateral asymptomatic plaques in patients with TIA/stroke who were imaged within 38 days, whereas MDCT detected larger vessel wall and LRNC volumes, regardless of time after symptoms. In view of the substantial overlap in measurements of both sides, it remains to be determined whether the differences we found will be clinically meaningful.
Figures

References
-
- Chaturvedi S, Bruno A, Feasby T, et al. , for the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Carotid endarterectomy: an evidence-based review—report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005;65:794–801 - PubMed
-
- Redgrave JN, Lovett JK, Gallagher PJ, et al. . Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford Plaque Study. Circulation 2006;113:2320–28 - PubMed
-
- Tawakol A, Migrino RQ, Bashian GG, et al. . In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–24 - PubMed
-
- de Weert TT, Ouhlous M, Meijering E, et al. . In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol 2006;26:2366–72 - PubMed
-
- Grant EG, Benson CB, Moneta GL, et al. . Carotid artery stenosis: gray-scale and Doppler US diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003;229:340–46 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
