High-resolution kinetics of cytokine signaling in human CD34/CD117-positive cells in unfractionated bone marrow
- PMID: 21330471
- PMCID: PMC3087539
- DOI: 10.1182/blood-2010-10-316224
High-resolution kinetics of cytokine signaling in human CD34/CD117-positive cells in unfractionated bone marrow
Abstract
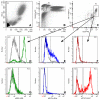
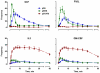
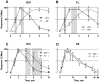
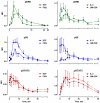
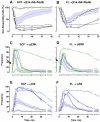
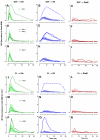
Cytokine-mediated phosphorylation of Erk (pErk), ribosomal S6 (pS6), and Stat5 (pStat5) in CD34(+)/CD117(+) blast cells in normal bone marrow from 9 healthy adult donors were analyzed over 60 minutes. Treatment with stem cell factor (SCF), Flt3-ligand (FL), IL-3, and GM-CSF and measurement by multiparametric flow cytometry yielded distinctive, highly uniform phosphoprotein kinetic profiles despite a diverse sample population. The correlated responses for SCF- and FL-stimulated pErk and pS6 were similar. Half the population phosphorylated Erk in response to SCF between 0.9 and 1.2 minutes, and S6 phosphorylation followed approximately a minute later (t½(pS6 rise) = 2.2-2.7 minutes). The FL response was equally fast but more variable (t½(pErk rise) = 0.9-1.3 minutes; t½(pS6 rise) = 2.5-3.5 minutes). Stat5 was not activated in 97% of the cells by either cytokine. IL-3 and GM-CSF were similar to each other with half of blast cells phosphorylating Stat5 and 15% to 20% responding through Erk and S6. Limited comparison with leukemic blasts confirmed universal abnormal signaling in AML that is significantly different from normal bone marrow blasts. These differences included sustained signals, a larger fraction of responding cells, and amplification of phosphorylation levels for at least one phosphoprotein. These data support the eventual use of this approach for disease diagnosis and monitoring.
Figures

References
-
- Chow S, Hedley D, Grom P, Magari R, Jacobberger JW, Shankey TV. Whole blood fixation and permeabilization protocol with red blood cell lysis for flow cytometry of intracellular phosphorylated epitopes in leukocyte subpopulations. Cytometry A. 2005;67(1):4–17. - PubMed
-
- Muller C, Bonmann M, Cassens U, Koch OM. Flow cytometric analysis of protein phosphorylation in the hematopoietic system. Leuk Lymphoma. 1998;29(3):351–360. - PubMed
-
- Fleisher TA, Dorman SE, Anderson JA, Vail M, Brown MR, Holland SM. Detection of intracellular phosphorylated STAT-1 by flow cytometry. Clin Immunol. 1999;90(3):425–430. - PubMed
-
- Chow S, Patel H, Hedley DW. Measurement of MAP kinase activation by flow cytometry using phospho-specific antibodies to MEK and ERK: potential for pharmacodynamic monitoring of signal transduction inhibitors. Cytometry. 2001;46(2):72–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

