cGMP signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes
- PMID: 21330599
- PMCID: PMC3083836
- DOI: 10.1161/CIRCRESAHA.110.230698
cGMP signals modulate cAMP levels in a compartment-specific manner to regulate catecholamine-dependent signaling in cardiac myocytes
Abstract
Rationale: cAMP and cGMP are intracellular second messengers involved in heart pathophysiology. cGMP can potentially affect cAMP signals via cGMP-regulated phosphodiesterases (PDEs).
Objective: To study the effect of cGMP signals on the local cAMP response to catecholamines in specific subcellular compartments.
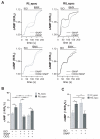
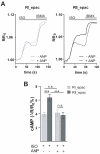
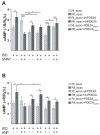
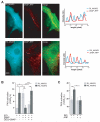
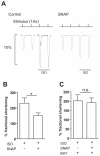
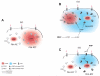
Methods and results: We used real-time FRET imaging of living rat ventriculocytes expressing targeted cAMP and cGMP biosensors to detect cyclic nucleotides levels in specific locales. We found that the compartmentalized, but not the global, cAMP response to isoproterenol is profoundly affected by cGMP signals. The effect of cGMP is to increase cAMP levels in the compartment where the protein kinase (PK)A-RI isoforms reside but to decrease cAMP in the compartment where the PKA-RII isoforms reside. These opposing effects are determined by the cGMP-regulated PDEs, namely PDE2 and PDE3, with the local activity of these PDEs being critically important. The cGMP-mediated modulation of cAMP also affects the phosphorylation of PKA targets and myocyte contractility.
Conclusions: cGMP signals exert opposing effects on local cAMP levels via different PDEs the activity of which is exerted in spatially distinct subcellular domains. Inhibition of PDE2 selectively abolishes the negative effects of cGMP on cAMP and may have therapeutic potential.
Figures
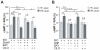
References
-
- El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev. 2009;14:225–241. - PubMed
-
- Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 2000;86:49–86. - PubMed
-
- Rosenkranz AC, Woods RL, Dusting GJ, Ritchie RH. Antihypertrophic actions of the natriuretic peptides in adult rat cardiomyocytes: importance of cyclic GMP. Cardiovasc Res. 2003;57:515–522. - PubMed
-
- Felker GM, Pang PS, Adams KF, Cleland JG, Cotter G, Dickstein K, Filippatos GS, Fonarow GC, Greenberg BH, Hernandez AF, Khan S, Komajda M, Konstam MA, Liu PP, Maggioni AP, Massie BM, McMurray JJ, Mehra M, Metra M, O’Connell J, O’Connor CM, Pina IL, Ponikowski P, Sabbah HN, Teerlink JR, Udelson JE, Yancy CW, Zannad F, Gheorghiade M. Clinical trials of pharmacological therapies in acute heart failure syndromes: lessons learned and directions forward. Circ Heart Fail. 2010;3:314–325. - PubMed
-
- Nony P, Boissel JP, Lievre M, Leizorovicz A, Haugh MC, Fareh S, de Breyne B. Evaluation of the effect of phosphodiesterase inhibitors on mortality in chronic heart failure patients. A meta-analysis. Eur J Clin Pharmacol. 1994;46:191–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

