Cyclophilin A promotes cardiac hypertrophy in apolipoprotein E-deficient mice
- PMID: 21330604
- PMCID: PMC3085960
- DOI: 10.1161/ATVBAHA.110.214601
Cyclophilin A promotes cardiac hypertrophy in apolipoprotein E-deficient mice
Abstract
Objective: Cyclophilin A (CyPA, encoded by Ppia) is a proinflammatory protein secreted in response to oxidative stress in mice and humans. We recently demonstrated that CyPA increased angiotensin II (Ang II)-induced reactive oxygen species (ROS) production in the aortas of apolipoprotein E (Apoe)-/- mice. In this study, we sought to evaluate the role of CyPA in Ang II-induced cardiac hypertrophy.
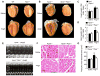
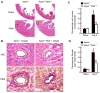
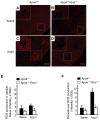
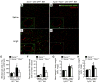
Methods and results: Cardiac hypertrophy was not significantly different between Ppia+/+ and Ppia-/- mice infused with Ang II (1000 ng/min per kg for 4 weeks). Therefore, we investigated the effect of CyPA under conditions of high ROS and inflammation using the Apoe-/- mice. In contrast to Apoe-/- mice, Apoe-/-Ppia-/- mice exhibited significantly less Ang II-induced cardiac hypertrophy. Bone marrow cell transplantation showed that CyPA in cells intrinsic to the heart plays an important role in the cardiac hypertrophic response. Ang II-induced ROS production, cardiac fibroblast proliferation, and cardiac fibroblast migration were markedly decreased in Apoe-/-Ppia-/- cardiac fibroblasts. Furthermore, CyPA directly induced the hypertrophy of cultured neonatal cardiac myocytes.
Conclusions: CyPA is required for Ang II-mediated cardiac hypertrophy by directly potentiating ROS production, stimulating the proliferation and migration of cardiac fibroblasts, and promoting cardiac myocyte hypertrophy.
Figures

References
-
- Izumo S, Aoki H. Calcineurin--the missing link in cardiac hypertrophy. Nat Med. 1998;4:661–662. - PubMed
-
- Jaffre F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay JM, Maroteaux L. Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ Res. 2009;104:113–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

