High pancreatic n-3 fatty acids prevent STZ-induced diabetes in fat-1 mice: inflammatory pathway inhibition
- PMID: 21330635
- PMCID: PMC3064083
- DOI: 10.2337/db10-0901
High pancreatic n-3 fatty acids prevent STZ-induced diabetes in fat-1 mice: inflammatory pathway inhibition
Abstract
Objective: Because of confounding factors, the effects of dietary n-3 polyunsaturated fatty acids (PUFA) on type 1 diabetes remain to be clarified. We therefore evaluated whether fat-1 transgenic mice, a well-controlled experimental model endogenously synthesizing n-3 PUFA, were protected against streptozotocin (STZ)-induced diabetes. We then aimed to elucidate the in vivo response at the pancreatic level.
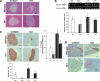
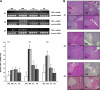
Research design and methods: β-Cell destruction was produced by multiple low-doses STZ (MLD-STZ). Blood glucose level, plasma insulin level, and plasma lipid analysis were then performed. Pancreatic mRNA expression of cytokines, the monocyte chemoattractant protein, and GLUT2 were evaluated as well as pancreas nuclear factor (NF)-κB p65 and inhibitor of κB (IκB) protein expression. Insulin and cleaved caspase-3 immunostaining and lipidomic analysis were performed in the pancreas.
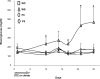
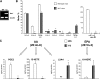
Results: STZ-induced fat-1 mice did not develop hyperglycemia compared with wild-type mice, and β-cell destruction was prevented as evidenced by lack of histological pancreatic damage or reduced insulin level. The prevention of β-cell destruction was associated with no proinflammatory cytokine induction (tumor necrosis factor-α, interleukin-1β, inducible nitric oxide synthase) in the pancreas, a decreased NF-κB, and increased IκB pancreatic protein expression. In the fat-1-treated mice, proinflammatory arachidonic-derived mediators as prostaglandin E₂ and 12-hydroxyeicosatetraenoic acid were decreased and the anti-inflammatory lipoxin A₄ was detected. Moreover, the 18-hydroxyeicosapentaenoic acid, precursor of the anti-inflammatory resolvin E1, was highly increased.
Conclusions: Collectively, these findings indicate that fat-1 mice were protected against MLD-STZ-induced diabetes and pointed out for the first time in vivo the beneficial effects of n-3 PUFA at the pancreatic level, on each step of the development of the pathology-inflammation, β-cell damage-through cytokine response and lipid mediator production.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

