Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register
- PMID: 21330639
- PMCID: PMC3048625
- DOI: 10.1136/ard.2010.139774
Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register
Abstract
Objective: To evaluate the effect of different concomitant disease modifying antirheumatic drugs (DMARDs) on the persistence with antitumour necrosis factor (anti-TNF) therapies in patients with rheumatoid arthritis (RA).
Method: This analysis included 10 396 patients with RA registered with the British Society for Rheumatology Biologics Register, a prospective observational cohort study, who were starting their first anti-TNF therapy and were receiving one of the following DMARD treatments at baseline: no DMARD (n=3339), methotrexate (MTX) (n=4418), leflunomide (LEF) (n=610), sulfasalazine (SSZ) (n=308), MTX+SSZ (n=902), MTX+ hydroxychloroquine (HCQ) (n=401) or MTX+SSZ+HCQ (n=418). Kaplan-Meier survival analysis was used to study the persistence with anti-TNF therapy in each DMARD subgroup up to 5 years. Multivariate Cox proportional hazard models, stratified by anti-TNF used and start year and adjusted for a number of potential confounders, were used to compare treatment persistence overall and according to the reason for discontinuation between each of the DMARD subgroups, using MTX as reference.
Results: One-year drug survival (95% CI) for the first anti-TNF therapy was 71% (71% to 72%) but this dropped to 42% (41% to 43%) at 5 years. Compared with MTX, patients receiving no DMARD, LEF or SSZ were more likely to discontinue their first anti-TNF therapy while patients receiving MTX in combination with other DMARDs showed better treatment persistence.
Conclusions: These results support the continued use of background DMARD combinations which include MTX. Consideration should be given to the discontinuation of LEF and SSZ monotherapy at the time anti-TNF therapies are started, with the possible exception of the SSZ+ETN combination.
Conflict of interest statement
Figures
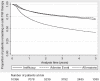
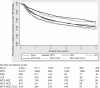
References
-
- Nixon R, Bansback N, Brennan A. The efficacy of inhibiting tumour necrosis factor alpha and interleukin 1 in patients with rheumatoid arthritis: a meta-analysis and adjusted indirect comparisons. Rheumatology (Oxford) 2007;46:1140–7 - PubMed
-
- Venkateshan SP, Sidhu S, Malhotra S, et al. Efficacy of biologicals in the treatment of rheumatoid arthritis. a meta-analysis. Pharmacology 2009;83:1–9 - PubMed
-
- Wiens A, Correr CJ, Pontarolo R, et al. A systematic review and meta-analysis of the efficacy and safety of etanercept for treating rheumatoid arthritis. Scand J Immunol 2009;70:337–44 - PubMed
-
- Hyrich KL, Symmons DP, Watson KD, et al. Comparison of the response to infliximab or etanercept monotherapy with the response to cotherapy with methotrexate or another disease-modifying antirheumatic drug in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006;54:1786–94 - PubMed

