Valsartan improves {beta}-cell function and insulin sensitivity in subjects with impaired glucose metabolism: a randomized controlled trial
- PMID: 21330640
- PMCID: PMC3064038
- DOI: 10.2337/dc10-2224
Valsartan improves {beta}-cell function and insulin sensitivity in subjects with impaired glucose metabolism: a randomized controlled trial
Abstract
Objective: Recently, the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research Trial demonstrated that treatment with the angiotensin receptor blocker (ARB) valsartan for 5 years resulted in a relative reduction of 14% in the incidence of type 2 diabetes in subjects with impaired glucose metabolism (IGM). We investigated whether improvements in β-cell function and/or insulin sensitivity underlie these preventive effects of the ARB valsartan in the onset of type 2 diabetes.
Research design and methods: In this randomized controlled, double-blind, two-center study, the effects of 26 weeks of valsartan (320 mg daily; n = 40) or placebo (n = 39) on β-cell function and insulin sensitivity were assessed in subjects with impaired fasting glucose and/or impaired glucose tolerance, using a combined hyperinsulinemic-euglycemic and hyperglycemic clamp with subsequent arginine stimulation and a 2-h 75-g oral glucose tolerance test (OGTT). Treatment effects were analyzed using ANCOVA, adjusting for center, glucometabolic status, and sex.
Results: Valsartan increased first-phase (P = 0.028) and second-phase (P = 0.002) glucose-stimulated insulin secretion compared with placebo, whereas the enhanced arginine-stimulated insulin secretion was comparable between groups (P = 0.25). In addition, valsartan increased the OGTT-derived insulinogenic index (representing first-phase insulin secretion after an oral glucose load; P = 0.027). Clamp-derived insulin sensitivity was significantly increased with valsartan compared with placebo (P = 0.049). Valsartan treatment significantly decreased systolic and diastolic blood pressure compared with placebo (P < 0.001). BMI remained unchanged in both treatment groups (P = 0.89).
Conclusions: Twenty-six weeks of valsartan treatment increased glucose-stimulated insulin release and insulin sensitivity in normotensive subjects with IGM. These findings may partly explain the beneficial effects of valsartan in the reduced incidence of type 2 diabetes.
Figures


 ) or placebo (PLB) (□). Data represent means ± SE or, in the case of nonnormally distributed data, medians (interquartile range).
) or placebo (PLB) (□). Data represent means ± SE or, in the case of nonnormally distributed data, medians (interquartile range).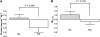
 ) or placebo (PLB) (□). Data represent means ± SE.
) or placebo (PLB) (□). Data represent means ± SE.References
-
- Shiuchi T, Iwai M, Li HS, et al. Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension 2004;43:1003–1010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

