Bis-indole derivatives for polysaccharide compositional analysis and chiral resolution of D-, L-monosaccharides by ligand exchange capillary electrophoresis using borate-cyclodextrin as a chiral selector
- PMID: 21330957
- PMCID: PMC6259635
- DOI: 10.3390/molecules16021682
Bis-indole derivatives for polysaccharide compositional analysis and chiral resolution of D-, L-monosaccharides by ligand exchange capillary electrophoresis using borate-cyclodextrin as a chiral selector
Abstract
A series of aldo-bis-indole derivatives (aldo-BINs) was prepared by aromatic C-alkylation reactions of aldoses and indole in acetic acid solution. Common monosaccharides such as glucose, mannose, galactose, fucose, xylose, rhamnose, ribose, arabinose and N-acetylglucosamine were smoothly derivatized to form the UV absorbing aldo-BINs. The use of a capillary electrophoretic method to separate these novel aldo-BIN derivatives was established. The capillary electrophoresis conditions were set by using borate buffer (100 mM) at high pH (pH 9.0). The limit of determination was assessed to be 25 nM. The enantioseparation of D, L-pairs of aldo-BINs based on chiral ligand-exchange capillary electrophoresis technology was also achieved by using modified hydroxypropyl-β-cyclodextrin as the chiral selector in the presence of borate buffer. This aldose labeling method was applied successfully to the compositional and configurational analysis of saccharides, exemplified by a rapid and efficient method to simultaneously analyze the composition and configuration of saccharides from the medicinal herbs Cordyceps sinensis and Dendrobium huoshanense.
Figures
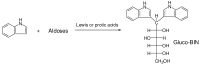


References
-
- Colombeau L., Traoré T., Compain P., Martin O.R. Metal-free one-pot oxidative amidation of aldoses with functionalized amines. J. Org. Chem. 2008;73:8647–8650. - PubMed
-
- Kamal A., Qureshi A.A. Synthesis of some substituted di-indolylmethanes in aqueous medium at room temperature. Tetrahedron. 1963;19:513–520. doi: 10.1016/S0040-4020(01)98540-0. - DOI
-
- Chen D., Yu L., Wang P.G. Lewis acid-catalyzed reactions in protic media. Lanthanide-catalyzed reactions of indoles with aldehydes or ketones. Tetrahedron Lett. 1996;37:4467–4470.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

