Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet
- PMID: 21331068
- PMCID: PMC3130193
- DOI: 10.1038/oby.2011.18
Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet
Abstract
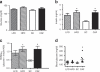
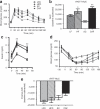
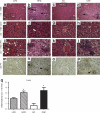
Obesity has reached epidemic proportions worldwide and reports estimate that American children consume up to 25% of calories from snacks. Several animal models of obesity exist, but studies are lacking that compare high-fat diets (HFD) traditionally used in rodent models of diet-induced obesity (DIO) to diets consisting of food regularly consumed by humans, including high-salt, high-fat, low-fiber, energy dense foods such as cookies, chips, and processed meats. To investigate the obesogenic and inflammatory consequences of a cafeteria diet (CAF) compared to a lard-based 45% HFD in rodent models, male Wistar rats were fed HFD, CAF or chow control diets for 15 weeks. Body weight increased dramatically and remained significantly elevated in CAF-fed rats compared to all other diets. Glucose- and insulin-tolerance tests revealed that hyperinsulinemia, hyperglycemia, and glucose intolerance were exaggerated in the CAF-fed rats compared to controls and HFD-fed rats. It is well-established that macrophages infiltrate metabolic tissues at the onset of weight gain and directly contribute to inflammation, insulin resistance, and obesity. Although both high fat diets resulted in increased adiposity and hepatosteatosis, CAF-fed rats displayed remarkable inflammation in white fat, brown fat and liver compared to HFD and controls. In sum, the CAF provided a robust model of human metabolic syndrome compared to traditional lard-based HFD, creating a phenotype of exaggerated obesity with glucose intolerance and inflammation. This model provides a unique platform to study the biochemical, genomic and physiological mechanisms of obesity and obesity-related disease states that are pandemic in western civilization today.
Figures




References
-
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. - PubMed
-
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303:242–249. - PubMed
-
- Klein JD, Dietz W. Childhood obesity: the new tobacco. Health Aff (Millwood) 2010;29:388–392. - PubMed
-
- Rothwell NJ, Stock MJ. Combined effects of cafeteria and tube-feeding on energy balance in the rat. Proc Nutr Soc. 1979;38:5A. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

