Engaging neuroscience to advance translational research in brain barrier biology
- PMID: 21331083
- PMCID: PMC3335275
- DOI: 10.1038/nrn2995
Engaging neuroscience to advance translational research in brain barrier biology
Abstract
The delivery of many potentially therapeutic and diagnostic compounds to specific areas of the brain is restricted by brain barriers, of which the most well known are the blood-brain barrier (BBB) and the blood-cerebrospinal fluid (CSF) barrier. Recent studies have shown numerous additional roles of these barriers, including an involvement in neurodevelopment, in the control of cerebral blood flow, and--when barrier integrity is impaired--in the pathology of many common CNS disorders such as Alzheimer's disease, Parkinson's disease and stroke.
Figures


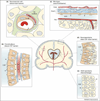

References
-
- Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–638. - PubMed
-
- Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. - PubMed
-
- Kortekaas R, et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. - PubMed
-
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

