Vascular complications following liver transplantation
- PMID: 21331133
- PMCID: PMC3036231
- DOI: 10.1055/s-2004-861556
Vascular complications following liver transplantation
Abstract
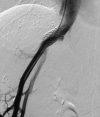
Vascular complications (stenosis or thrombosis of the hepatic artery, portal vein or hepatic vein) are a relatively common occurrence following liver transplantation. Routine screening with ultrasound is critical to early detection of these complications. Careful application of standard interventional techniques (diagnostic catheter angiography, balloon angioplasty with selective stenting) may be used to confirm the ultrasound findings, treat the underlying lesions, and contribute to long-term graft survival.
Keywords: Liver transplantation; balloon angioplasty; hepatic artery; hepatic vein; portal vein.
Figures





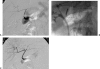
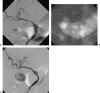
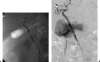
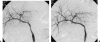
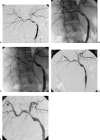
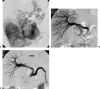

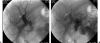
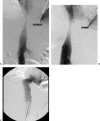

Similar articles
-
Long-term venous complications after full-size and segmental pediatric liver transplantation.Ann Surg. 2002 Nov;236(5):658-66. doi: 10.1097/00000658-200211000-00017. Ann Surg. 2002. PMID: 12409673 Free PMC article.
-
Intraoperative Doppler ultrasound in liver transplantation.Clin Transplant. 1998 Aug;12(4):292-9. Clin Transplant. 1998. PMID: 9686322
-
Major vascular complications in living-donor liver transplant recipients: single center team experience.Exp Clin Transplant. 2015 Apr;13 Suppl 1:64-70. Exp Clin Transplant. 2015. PMID: 25894130
-
[Application of interventional radiology for stenosis of vascular anastomosis in living-donor liver transplantation].Nihon Geka Gakkai Zasshi. 2004 Jun;105(6):364-8. Nihon Geka Gakkai Zasshi. 2004. PMID: 15224618 Review. Japanese.
-
Management of portal venous complications after liver transplantation.Tech Vasc Interv Radiol. 2007 Sep;10(3):233-9. doi: 10.1053/j.tvir.2007.09.017. Tech Vasc Interv Radiol. 2007. PMID: 18086428 Review.
Cited by
-
The Role of Interventional Radiology in Treating Complications following Liver Transplantation.ISRN Hepatol. 2012 Dec 3;2013:696794. doi: 10.1155/2013/696794. eCollection 2013. ISRN Hepatol. 2012. PMID: 27335821 Free PMC article. Review.
-
Iatrogenic-related transplant injuries: the role of the interventional radiologist.Semin Intervent Radiol. 2015 Jun;32(2):133-55. doi: 10.1055/s-0035-1549842. Semin Intervent Radiol. 2015. PMID: 26038621 Free PMC article. Review.
-
Outcome of prolonged acute vena cava occlusion after iatrogenic transection and repair in a dog.Can Vet J. 2017 Aug;58(8):845-850. Can Vet J. 2017. PMID: 28761192 Free PMC article.
References
-
- Orons P D, Zajko A B, Bron K M, et al. Hepatic artery angioplasty after liver transplantation: experience in 21 allografts. J Vasc Interv Radiol. 1995;6:523–529. - PubMed
-
- Soin A S, Friend P J, Rasmussen A, et al. Donor arterial variations in liver transplantation: management and outcome of 527 consecutive grafts. Br J Surg. 1996;83:637–641. - PubMed
-
- Neuhaus P, Platz K P. Liver transplantation: newer surgical approaches. Baillieres Clin Gastroenterol. 1994;8:481–493. - PubMed
-
- Cappadonna C R, Johnson L B, Lu A D, et al. Outcome of extra-anatomic vascular reconstruction in orthotopic liver transplantation. Am J Surg. 2001;182:147–150. - PubMed
-
- Figueras J, Pares D, Aranda H, et al. Results of using the recipient's splenic artery for arterial reconstruction in liver transplantation in 23 patients. Transplantation. 1997;64:655–658. - PubMed

