A high-performance liquid chromatography:chemiluminescence method for potential determination of vardenafil in dietary supplement
- PMID: 21331172
- PMCID: PMC3034921
- DOI: 10.1155/2011/982186
A high-performance liquid chromatography:chemiluminescence method for potential determination of vardenafil in dietary supplement
Abstract
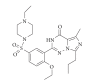
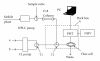
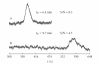
A flow method of high-performance liquid chromatography (HPLC) seperation and chemiluminescence (CL) detection for sensitive vardenafil analysis in dietary supplements was developed. The vardenafil separation was achieved on a C18 column at 30°C using ethanol-H(3)PO(4) and ethylenediaminetetraacetic acid disodium salt (Na(2)EDTA) aqueous solution (25 : 75, v/v%) as mobile phase. The followed continuous CL detection was conducted based on the strong CL enhancement by the presence of vardenafil to luminol-K(3)Fe(CN)(6) reaction in alkaline medium. At the flow rate of 0.8 mL/min, the vardenafil retention time (t(R)) was 6.4 min. Factors that affected the HPLC resolution and CL detection were studied and optimized. The calibration curve obtained for vardenafil standard was linear in concentration range of 8.0 × 10(-7) ~ 1.0 × 10(-4) mol/L. The relative standard deviations (RSD) of intraday and interday precision were less than 3.5%. The proposed method was applied to the vardenafil determination in oral liquid, wine, and capsule samples.
Figures

References
-
- Zhu X, Xiao S, Chen BO, et al. Simultaneous determination of sildenafil, vardenafil and tadalafil as forbidden components in natural dietary supplements for male sexual potency by high-performance liquid chromatography-electrospray ionization mass spectrometry. Journal of Chromatography A. 2005;1066(1-2):89–95. - PubMed
-
- Cheng CL, Kang GJ, Chou CH. Development and validation of a high-performance liquid chromatographic method using fluorescence detection for the determination of vardenafil in small volumes of rat plasma and bile. Journal of Chromatography A. 2007;1154(1-2):222–229. - PubMed
-
- Uslu B, Dogan B, Özkan SA, Aboul-Enein HY. Electrochemical behavior of vardenafil on glassy carbon electrode: determination in tablets and human serum. Analytica Chimica Acta. 2005;552(1-2):127–134.
-
- Gratz SR, Flurer CL, Wolnik KA. Analysis of undeclared synthetic phosphodiesterase-5 inhibitors in dietary supplements and herbal matrices by LC-ESI-MS and LC-UV. Journal of Pharmaceutical and Biomedical Analysis. 2004;36(3):525–533. - PubMed
-
- Rajagopalan P, Mazzu A, Xia C, Dawkins R, Sundaresan P. Effect of high-fat breakfast and moderate-fat evening meal on the pharmacokinetics of vardenafil, an oral phosphodiesterase-5 inhibitor for the treatment of erectile dysfunction. Journal of Clinical Pharmacology. 2003;43(3):260–267. - PubMed
LinkOut - more resources
Full Text Sources
