Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers
- PMID: 21331354
- PMCID: PMC3039422
Role of fatty acid synthase in gemcitabine and radiation resistance of pancreatic cancers
Abstract
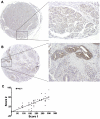
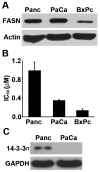
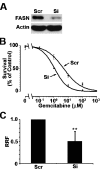
Human fatty acid synthase (FASN) is a homo-dimeric protein with multi-enzymatic activity responsible for the synthesis of palmitate. FASN expression has been found to be up-regulated in multiple types of human cancers and its expression correlates with poor prognosis possibly by causing treatment resistance. In this study, we tested if FASN expression is up-regulated in human pancreatic cancers and if its higher expression level in pancreatic cancers causes intrinsic resistance to gemcitabine and radiation. We found that FASN expression is significantly up-regulated in human pancreatic cancer tissues without any correlation to age, sex, race, and tumor stage. Knocking down or over-expressing FASN significantly down- or up-regulate resistance of pancreatic cancer cell lines to both gemcitabine and radiation treatments. These findings imply that the elevated FASN expression in pancreatic cancers may contribute to unsuccessful treatments of pancreatic cancers by causing intrinsic resistance to both chemotherapy and radiation therapy.
Keywords: Human fatty acid synthase (FASN); gemcitabine; palmitate; pancreatic cancers; radiation treatments; treatment resistance.
Figures


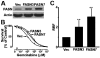

References
-
- Alo PL, Amini M, Piro F, Pizzuti L, Sebastiani V, Botti C, Murari R, Zotti G, Di Tondo U. Immu-nohistochemical expression and prognostic significance of fatty acid synthase in pancreatic carcinoma. Anticancer Res. 2007;27:2523–2527. - PubMed
-
- Witkiewicz AK, Nguyen KH, Dasgupta A, Kennedy EP, Yeo CJ, Lisanti MP, Brody JR. Co-expression of fatty acid synthase and caveolin-1 in pancreatic ductal adenocarcinoma: implications for tumor progression and clinical outcome. Cell Cycle. 2008;7:3021–3025. - PubMed
-
- Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol. 1996;27:917–921. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
