Diffusion as a probe of peptide-induced membrane domain formation
- PMID: 21332237
- PMCID: PMC3062684
- DOI: 10.1021/bi102068j
Diffusion as a probe of peptide-induced membrane domain formation
Abstract
Recently, we have shown that association with an antimicrobial peptide (AMP) can drastically alter the diffusion behavior of the constituent lipids in model membranes (Biochemistry 49, 4672-4678). In particular, we found that the diffusion time of a tracer fluorescent lipid through a confocal volume measured via fluorescence correlation spectroscopy (FCS) is distributed over a wide range of time scales, indicating the formation of stable and/or transient membrane species that have different mobilities. A simple estimate, however, suggested that the slow diffusing species are too large to be attributed to AMP oligomers or pores that are tightly bound to a small number of lipids. Thus, we tentatively ascribed them to membrane domains and/or clusters that possess distinctively different diffusion properties. In order to further substantiate our previous conjecture, herein we study the diffusion behavior of the membrane-bound peptide molecules using the same AMPs and model membranes. Our results show, in contrast to our previous findings, that the diffusion times of the membrane-bound peptides exhibit a much narrower distribution that is more similar to that of the lipids in peptide-free membranes. Thus, taken together, these results indicate that while AMP molecules prompt domain formation in membranes, they are not tightly associated with the lipid domains thus formed. Instead, they are likely located at the boundary regions separating various domains and acting as mobile fences.
Figures




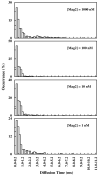

References
-
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 1995;3:238–250. - PubMed
-
- Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membrane Biol. 1997;156:197–211. - PubMed
-
- Bechinger B. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim Biophys Acta. 1999;1462:157–183. - PubMed
-
- Sitaram N, Nagaraj R. Interaction of antimicrobial peptides with biological and model membranes: structural and charge requirements for activity. Biochim Biophys Acta. 1999;1462:29–54. - PubMed
-
- Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

