The cyanobacterial circadian system: from biophysics to bioevolution
- PMID: 21332358
- PMCID: PMC3093959
- DOI: 10.1146/annurev-biophys-042910-155317
The cyanobacterial circadian system: from biophysics to bioevolution
Abstract
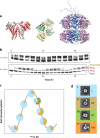
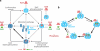
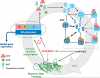
Recent studies have unveiled the molecular machinery responsible for the biological clock in cyanobacteria and found that it exerts pervasive control over cellular processes including global gene expression. Indeed, the entire chromosome undergoes daily cycles of topology/compaction! The circadian system comprises both a posttranslational oscillator (PTO) and a transcriptional/translational feedback loop (TTFL). The PTO can be reconstituted in vitro with three purified proteins (KaiA, KaiB, and KaiC) and ATP. These are the only circadian proteins for which high-resolution structures are available. Phase in this nanoclockwork has been associated with key phosphorylations of KaiC. Structural considerations illuminate the mechanism by which the KaiABC oscillator ratchets unidirectionally. Models of the complete in vivo system have important implications for our understanding of circadian clocks in higher organisms, including mammals. The conjunction of structural, biophysical, and biochemical approaches to this system has brought our understanding of the molecular mechanisms of biological timekeeping to an unprecedented level.
Figures



References
-
- Akiyama S, Nohara A, Ito K, Maéda Y. Assembly and disassembly dynamics of the cyanobacterial periodosome. Mol. Cell. 2008;29:703–16. - PubMed
-
- Andersson CR, Tsinoremas NF, Shelton J, Lebedeva NV, Yarrow J, et al. Application of bioluminescence to the study of circadian rhythms in cyanobacteria. Methods Enzymol. 2000;305:527–42. - PubMed
-
- Angermayr SA, Hellingwerf KJ, Lindblad P, de Mattos MJ. Energy biotechnology with cyanobacteria. Curr. Opin. Biotechnol. 2009;20:257–63. - PubMed
-
- Atsumi S, Higashide W, Liao JC. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 2009;27:1177–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

