Development of an erythropoietin prescription simulator to improve abilities for the prescription of erythropoietin stimulating agents: is it feasible?
- PMID: 21332992
- PMCID: PMC3055807
- DOI: 10.1186/1471-2369-12-11
Development of an erythropoietin prescription simulator to improve abilities for the prescription of erythropoietin stimulating agents: is it feasible?
Abstract
Background: The increasing use of erythropoietins with long half-lives and the tendency to lengthen the administration interval to monthly injections call for raising awareness on the pharmacokinetics and risks of new erythropoietin stimulating agents (ESA). Their pharmacodynamic complexity and individual variability limit the possibility of attaining comprehensive clinical experience. In order to help physicians acquiring prescription abilities, we have built a prescription computer model to be used both as a simulator and education tool.
Methods: The pharmacokinetic computer model was developed using Visual Basic on Excel and tested with 3 different ESA half-lives (24, 48 and 138 hours) and 2 administration intervals (weekly vs. monthly). Two groups of 25 nephrologists were exposed to the six randomised combinations of half-life and administration interval. They were asked to achieve and maintain, as precisely as possible, the haemoglobin target of 11-12 g/dL in a simulated naïve patient. Each simulation was repeated twice, with or without randomly generated bleeding episodes.
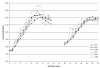
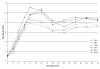
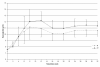
Results: The simulation using an ESA with a half-life of 138 hours, administered monthly, compared to the other combinations of half-lives and administration intervals, showed an overshooting tendency (percentages of Hb values > 13 g/dL 15.8 ± 18.3 vs. 6.9 ± 12.2; P < 0.01), which was quickly corrected with experience. The prescription ability appeared to be optimal with a 24 hour half-life and weekly administration (ability score indexing values in the target 1.52 ± 0.70 vs. 1.24 ± 0.37; P < 0.05). The monthly prescription interval, as suggested in the literature, was accompanied by less therapeutic adjustments (4.9 ± 2.2 vs. 8.2 ± 4.9; P < 0.001); a direct correlation between haemoglobin variability and number of therapy modifications was found (P < 0.01).
Conclusions: Computer-based simulations can be a useful tool for improving ESA prescription abilities among nephrologists by raising awareness about the pharmacokinetic characteristics of the various ESAs and recognizing the factors that influence haemoglobin variability.
Figures

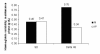



References
-
- Macdougall IC, Gray SJ, Elston O, Breen C, Jenkins B, Browne J, Egrie J. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392–2395. - PubMed
-
- Macdougall IC, Robson R, Opatrna S, Liogier X, Pannier A, Jordan P, Dougherty FC, Reigner B. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:1211–1215. doi: 10.2215/CJN.00730306. - DOI - PubMed
-
- Macdougall IC. CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep. 2005;4:436–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

