Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation--a perspective
- PMID: 21333360
- PMCID: PMC7127151
- DOI: 10.1016/j.jri.2011.01.010
Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation--a perspective
Abstract
It is self-evident that infection and inflammation in the reproductive tract can inhibit male fertility, but the observation that fertility may also be compromised by systemic inflammation and disease is more difficult to explain. Recent studies implicating microbial pattern-recognition receptors, such as the Toll-like receptors (TLRs), as well as inflammatory cytokines and their signalling pathways, in testicular function have cast new light on this mysterious link between infection/inflammation and testicular dysfunction. It is increasingly evident that signalling pathways normally involved in controlling inflammation play fundamental roles in regulating Sertoli cell activity and responses to reproductive hormones, in addition to promoting immune responses within the testis. Many of the negative effects of inflammation on spermatogenesis may be attributed to elevated production of inflammation-related gene products within the circulation and the testis, which subsequently exert disruptive effects on spermatogenic cell development and survival, as well as the ability of the Sertoli cells to provide support for spermatogenesis. These interactions have important implications for testicular dysfunction and disease, and may eventually provide new opportunities for therapeutic interventions.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures
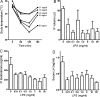
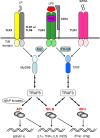
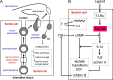
References
-
- Aly H.A., Lightfoot D.A., El-Shemy H.A. Bacterial lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. Toxicol. In Vitro. 2010;24:1266–1272. - PubMed
-
- Aratchige P.E., McIntyre P.B., Quinn H.E., Gilbert G.L. Recent increases in mumps incidence in Australia: the “forgotten’ age group in the 1998 Australian Measles Control Campaign. Med. J. Aust. 2008;189:434–437. - PubMed
-
- Aubry F., Habasque C., Satie A.P., Jégou B., Samson M. Expression and regulation of the CC-chemokine monocyte chemoattractant protein-1 in rat testicular cells in primary culture. Biol. Reprod. 2000;62:1427–1435. - PubMed
-
- Baker H.W. Reproductive effects of nontesticular illness. Endocrinol. Metab. Clin. North Am. 1998;27:831–850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

