Mechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface charge
- PMID: 21333757
- PMCID: PMC3138823
- DOI: 10.1016/j.nano.2011.01.011
Mechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface charge
Abstract
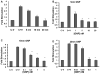
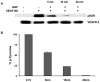
Discovering therapeutic inorganic nanoparticles (NPs) is evolving as an important area of research in the emerging field of nanomedicine. Recently, we reported the anti-angiogenic property of gold nanoparticles (GNPs): It inhibits the function of pro-angiogenic heparin-binding growth factors (HB-GFs), such as vascular endothelial growth factor 165 (VEGF165) and basic fibroblast growth factor (bFGF), etc. However, the mechanism through which GNPs imparts such an effect remains to be investigated. Using GNPs of different sizes and surface charges, we demonstrate here that a naked GNP surface is required and core size plays an important role to inhibit the function of HB-GFs and subsequent intracellular signaling events. We also demonstrate that the inhibitory effect of GNPs is due to the change in HB-GFs conformation/configuration (denaturation) by the NPs, whereas the conformations of non-HB-GFs remain unaffected. We believe that this significant study will help structure-based design of therapeutic NPs to inhibit the functions of disease-causing proteins.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
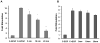


Similar articles
-
Gold Nanocrystals with Well-Defined Crystallographic {111} Facets Suppress Pathological Neovascularization.J Biomed Nanotechnol. 2016 Jul;12(7):1520-26. doi: 10.1166/jbn.2016.2260. J Biomed Nanotechnol. 2016. PMID: 29337491
-
Antiangiogenic properties of gold nanoparticles.Clin Cancer Res. 2005 May 1;11(9):3530-4. doi: 10.1158/1078-0432.CCR-04-2482. Clin Cancer Res. 2005. PMID: 15867256
-
Manipulation of in vitro angiogenesis using peptide-coated gold nanoparticles.ACS Nano. 2013 Jun 25;7(6):5628-36. doi: 10.1021/nn402111z. Epub 2013 May 28. ACS Nano. 2013. PMID: 23713973
-
Gold Nanoparticle-Induced Cell Death and Potential Applications in Nanomedicine.Int J Mol Sci. 2018 Mar 7;19(3):754. doi: 10.3390/ijms19030754. Int J Mol Sci. 2018. PMID: 29518914 Free PMC article. Review.
-
Anti-FGF2 approaches as a strategy to compensate resistance to anti-VEGF therapy: long-pentraxin 3 as a novel antiangiogenic FGF2-antagonist.Eur Cytokine Netw. 2009 Dec;20(4):225-34. doi: 10.1684/ecn.2009.0175. Eur Cytokine Netw. 2009. PMID: 20167562 Review.
Cited by
-
Nanotechnology for angiogenesis: opportunities and challenges.Chem Soc Rev. 2020 Jul 21;49(14):5008-5057. doi: 10.1039/c8cs01021h. Epub 2020 Jun 15. Chem Soc Rev. 2020. PMID: 32538379 Free PMC article. Review.
-
Effective treatment of intractable diseases using nanoparticles to interfere with vascular supply and angiogenic process.Eur J Med Res. 2022 Nov 4;27(1):232. doi: 10.1186/s40001-022-00833-6. Eur J Med Res. 2022. PMID: 36333816 Free PMC article. Review.
-
Inhibiting the growth of pancreatic adenocarcinoma in vitro and in vivo through targeted treatment with designer gold nanotherapeutics.PLoS One. 2013;8(3):e57522. doi: 10.1371/journal.pone.0057522. Epub 2013 Mar 6. PLoS One. 2013. PMID: 23483913 Free PMC article.
-
Gold nanoparticles attenuate albuminuria by inhibiting podocyte injury in a rat model of diabetic nephropathy.Drug Deliv Transl Res. 2020 Feb;10(1):216-226. doi: 10.1007/s13346-019-00675-6. Drug Deliv Transl Res. 2020. PMID: 31637677 Free PMC article.
-
Role of Sirtuins in Tumor Angiogenesis.Front Oncol. 2020 Jan 17;9:1516. doi: 10.3389/fonc.2019.01516. eCollection 2019. Front Oncol. 2020. PMID: 32010617 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

