Cowpox virus inhibits human dendritic cell immune function by nonlethal, nonproductive infection
- PMID: 21334039
- PMCID: PMC3694803
- DOI: 10.1016/j.virol.2011.01.024
Cowpox virus inhibits human dendritic cell immune function by nonlethal, nonproductive infection
Abstract
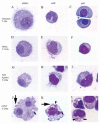
Orthopoxviruses encode multiple proteins that modulate host immune responses. We determined whether cowpox virus (CPXV), a representative orthopoxvirus, modulated innate and acquired immune functions of human primary myeloid DCs and plasmacytoid DCs and monocyte-derived DCs (MDDCs). A CPXV infection of DCs at a multiplicity of infection of 10 was nonproductive, altered cellular morphology, and failed to reduce cell viability. A CPXV infection of DCs did not stimulate cytokine or chemokine secretion directly, but suppressed toll-like receptor (TLR) agonist-induced cytokine secretion and a DC-stimulated mixed leukocyte reaction (MLR). LPS-stimulated NF-κB nuclear translocation and host cytokine gene transcription were suppressed in CPXV-infected MDDCs. Early viral immunomodulatory genes were upregulated in MDDCs, consistent with early DC immunosuppression via synthesis of intracellular viral proteins. We conclude that a nonproductive CPXV infection suppressed DC immune function by synthesizing early intracellular viral proteins that suppressed DC signaling pathways.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
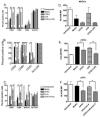
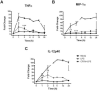

References
-
- Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166(3):1452–6. - PubMed
-
- Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96(3):1039–46. - PubMed
-
- Assarsson E, Greenbaum JA, Sundstrom M, Schaffer L, Hammond JA, Pasquetto V, Oseroff C, Hendrickson RC, Lefkowitz EJ, Tscharke DC, Sidney J, Grey HM, Head SR, Peters B, Sette A. Kinetic analysis of a complete poxvirus transcriptome reveals an immediate-early class of genes. Proc Natl Acad Sci U S A. 2008;105(6):2140–5. - PMC - PubMed
-
- Bauer M, Redecke V, Ellwart JW, Scherer B, Kremer JP, Wagner H, Lipford GB. Bacterial CpG-DNA triggers activation and maturation of human CD11c-, CD123+ dendritic cells. J Immunol. 2001;166(8):5000–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

