Long-term plasticity at inhibitory synapses
- PMID: 21334194
- PMCID: PMC3092861
- DOI: 10.1016/j.conb.2011.01.006
Long-term plasticity at inhibitory synapses
Abstract
Experience-dependent modifications of neural circuits and function are believed to heavily depend on changes in synaptic efficacy such as LTP/LTD. Hence, much effort has been devoted to elucidating the mechanisms underlying these forms of synaptic plasticity. Although most of this work has focused on excitatory synapses, it is now clear that diverse mechanisms of long-term inhibitory plasticity have evolved to provide additional flexibility to neural circuits. By changing the excitatory/inhibitory balance, GABAergic plasticity can regulate excitability, neural circuit function and ultimately, contribute to learning and memory, and neural circuit refinement. Here we discuss recent advancements in our understanding of the mechanisms and functional relevance of GABAergic inhibitory synaptic plasticity.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
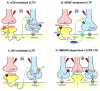

References
-
-
Woodin MA, Maffei A, editors. Inhibitory Synaptic Plasticity. edn 1st Springer; New York Dordrecht Heidelberg London: 2011. (••) This book summarizes most recent advancements on GABAergic plasticity in the mammalian brain. A must read to all those interested in inhibitory synapses and brain function under normal and pathological conditions.
-
-
- Gaiarsa J, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. - PubMed
-
-
Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. (••) A comprehensive review of the various retrograde signaling mechanisms involved in the regulation of excitatory and inhibitory synaptic transmission.
-
-
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

