Development of monoclonal antibodies (MAbs) to feline interferon (fIFN)-γ as tools to evaluate cellular immune responses to feline infectious peritonitis virus (FIPV)
- PMID: 21334239
- PMCID: PMC7129491
- DOI: 10.1016/j.jfms.2011.01.008
Development of monoclonal antibodies (MAbs) to feline interferon (fIFN)-γ as tools to evaluate cellular immune responses to feline infectious peritonitis virus (FIPV)
Abstract
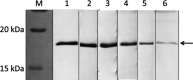
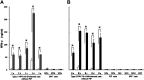
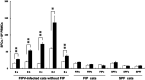
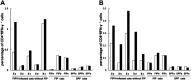
Feline infectious peritonitis virus (FIPV) can cause a lethal disease in cats, feline infectious peritonitis (FIP). The antibody-dependent enhancement (ADE) of FIPV infection has been recognised in experimentally infected cats, and cellular immunity is considered to play an important role in preventing the onset of FIP. To evaluate the importance of cellular immunity for FIPV infection, monoclonal antibodies (MAbs) against feline interferon (fIFN)-γ were first created to establish fIFN-γ detection systems using the MAbs. Six anti-fIFN-γ MAbs were created. Then, the difference in epitope which those MAbs recognise was demonstrated by competitive enzyme-linked immunosorbent assay (ELISA) and IFN-γ neutralisation tests. Detection systems for fIFN-γ (sandwich ELISA, ELISpot assay, and two-colour flow cytometry) were established using anti-fIFN-γ MAbs that recognise different epitopes. In all tests, fIFN-γ production from peripheral blood mononuclear cells (PBMCs) obtained from cats experimentally infected with an FIPV isolate that did not develop the disease was significantly increased by heat-inactivated FIPV stimulation in comparison with medium alone. Especially, CD8(+)fIFN-γ(+) cells, but not CD4(+)fIFN-γ(+) cells, were increased. In contrast, fIFN-γ production from PBMCs isolated from cats that had developed FIP and specific pathogen-free (SPF) cats was not increased by heat-inactivated FIPV stimulation. These results suggest that cellular immunity plays an important role in preventing the development of FIP. Measurement of fIFN-γ production with the anti-fIFN-γ MAbs created in this study appeared to be useful in evaluating cellular immunity in cats.
Copyright © 2011 ISFM and AAFP. Published by Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Characterization of antiviral T cell responses during primary and secondary challenge of laboratory cats with feline infectious peritonitis virus (FIPV).BMC Vet Res. 2019 May 22;15(1):165. doi: 10.1186/s12917-019-1909-6. BMC Vet Res. 2019. PMID: 31118053 Free PMC article.
-
Screening and identification of T helper 1 and linear immunodominant antibody-binding epitopes in the spike 2 domain and the nucleocapsid protein of feline infectious peritonitis virus.Vaccine. 2011 Feb 17;29(9):1791-800. doi: 10.1016/j.vaccine.2010.12.106. Epub 2011 Jan 7. Vaccine. 2011. PMID: 21216312 Free PMC article.
-
Development of antibodies to feline IFN-gamma as tools to elucidate the cellular immune responses to FeLV.J Immunol Methods. 2003 Aug;279(1-2):69-78. doi: 10.1016/s0022-1759(03)00244-8. J Immunol Methods. 2003. PMID: 12969548 Free PMC article.
-
Strategies for combating FIPV infection: antiviral agents and vaccines.Res Vet Sci. 2025 Aug;192:105709. doi: 10.1016/j.rvsc.2025.105709. Epub 2025 May 27. Res Vet Sci. 2025. PMID: 40446698 Review.
-
A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination.Vet Microbiol. 1993 Jul;36(1-2):1-37. doi: 10.1016/0378-1135(93)90126-r. Vet Microbiol. 1993. PMID: 8236772 Free PMC article. Review.
Cited by
-
Characterization of antiviral T cell responses during primary and secondary challenge of laboratory cats with feline infectious peritonitis virus (FIPV).BMC Vet Res. 2019 May 22;15(1):165. doi: 10.1186/s12917-019-1909-6. BMC Vet Res. 2019. PMID: 31118053 Free PMC article.
-
An update on feline infectious peritonitis: virology and immunopathogenesis.Vet J. 2014 Aug;201(2):123-32. doi: 10.1016/j.tvjl.2014.04.017. Epub 2014 May 2. Vet J. 2014. PMID: 24837550 Free PMC article. Review.
-
In Vitro Effects of Doxycycline on Replication of Feline Coronavirus.Pathogens. 2021 Mar 7;10(3):312. doi: 10.3390/pathogens10030312. Pathogens. 2021. PMID: 33799985 Free PMC article.
-
Screening and identification of T helper 1 and linear immunodominant antibody-binding epitopes in spike 1 domain and membrane protein of feline infectious peritonitis virus.Vaccine. 2014 Apr 1;32(16):1834-40. doi: 10.1016/j.vaccine.2014.01.074. Epub 2014 Feb 11. Vaccine. 2014. PMID: 24530149 Free PMC article.
-
Generating and evaluating type I interferon receptor-deficient and feline TMPRSS2-expressing cells for propagating serotype I feline infectious peritonitis virus.Virology. 2019 Nov;537:226-236. doi: 10.1016/j.virol.2019.08.030. Epub 2019 Aug 30. Virology. 2019. PMID: 31539770 Free PMC article.
References
-
- Pedersen N.C., Boyle J.F., Floyd K., Fudge A., Barker J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis, Am J Vet Res 42, 1981, 368–377. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

