Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies
- PMID: 21334333
- PMCID: PMC4404515
- DOI: 10.1053/j.gastro.2011.02.018
Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies
Abstract
Background & aims: Glucagon-like peptide-1-based therapy is gaining widespread use for type 2 diabetes, although there are concerns about risks for pancreatitis and pancreatic and thyroid cancers. There are also concerns that dipeptidyl peptidase-4 inhibitors could cause cancer, given their effects on immune function.
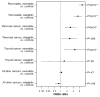
Methods: We examined the US Food and Drug Administration's database of reported adverse events for those associated with the dipeptidyl peptidase-4 inhibitor sitagliptin and the glucagon-like peptide-1 mimetic exenatide, from 2004-2009; data on adverse events associated with 4 other medications were compared as controls. The primary outcomes measures were rates of reported pancreatitis, pancreatic and thyroid cancer, and all cancers associated with sitagliptin or exenatide, compared with other therapies.
Results: Use of sitagliptin or exenatide increased the odds ratio for reported pancreatitis 6-fold as compared with other therapies (P<2×10(-16)). Pancreatic cancer was more commonly reported among patients who took sitagliptin or exenatide as compared with other therapies (P<.008, P<9×10(-5)). All other cancers occurred similarly among patients who took sitagliptin compared with other therapies (P=.20).
Conclusions: These data are consistent with case reports and animal studies indicating an increased risk for pancreatitis with glucagon-like peptide-1-based therapy. The findings also raise caution about the potential long-term actions of these drugs to promote pancreatic cancer.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest
The authors disclose no conflicts.
Figures
Comment in
-
GLP-1-based therapies: the dilemma of uncertainty.Gastroenterology. 2011 Jul;141(1):20-3. doi: 10.1053/j.gastro.2011.05.019. Gastroenterology. 2011. PMID: 21723985 No abstract available.
-
Pro- or anti-inflammatory properties of the adipokine dipeptidyl peptidase-4?Gastroenterology. 2011 Dec;141(6):e17. doi: 10.1053/j.gastro.2011.08.053. Epub 2011 Oct 27. Gastroenterology. 2011. PMID: 22036848 No abstract available.
References
-
- Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev. 2009;5:262–269. - PubMed
-
- Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Exp Rev Mol Med. 2010;12:e1. - PubMed
-
- Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. - PubMed
-
- Deacon CF. Incretin-based treatment of type 2 diabetes: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2007;9(Suppl 1):23–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical