Concentration of the antibacterial precursor thiocyanate in cystic fibrosis airway secretions
- PMID: 21334431
- PMCID: PMC3070840
- DOI: 10.1016/j.freeradbiomed.2011.02.013
Concentration of the antibacterial precursor thiocyanate in cystic fibrosis airway secretions
Abstract
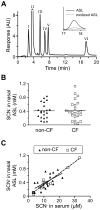
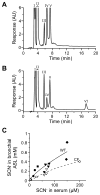
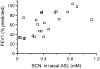
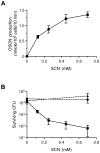
A recently discovered enzyme system produces antibacterial hypothiocyanite (OSCN(-)) in the airway lumen by oxidizing the secreted precursor thiocyanate (SCN(-)). Airway epithelial cultures have been shown to secrete SCN(-) in a CFTR-dependent manner. Thus, reduced SCN(-) availability in the airway might contribute to the pathogenesis of cystic fibrosis (CF), a disease caused by mutations in the CFTR gene and characterized by an airway host defense defect. We tested this hypothesis by analyzing the SCN(-) concentration in the nasal airway surface liquid (ASL) of CF patients and non-CF subjects and in the tracheobronchial ASL of CFTR-ΔF508 homozygous pigs and control littermates. In the nasal ASL, the SCN(-) concentration was ~30-fold higher than in serum independent of the CFTR mutation status of the human subject. In the tracheobronchial ASL of CF pigs, the SCN(-) concentration was somewhat reduced. Among human subjects, SCN(-) concentrations in the ASL varied from person to person independent of CFTR expression, and CF patients with high SCN(-) levels had better lung function than those with low SCN(-) levels. Thus, although CFTR can contribute to SCN(-) transport, it is not indispensable for the high SCN(-) concentration in ASL. The correlation between lung function and SCN(-) concentration in CF patients may reflect a beneficial role for SCN(-).
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Gerson C, Sabater J, Scuri M, Torbati A, Coffey R, Abraham JW, Lauredo I, Forteza R, Wanner A, Salathe M, Abraham WM, Conner GE. The lactoperoxidase system functions in bacterial clearance of airways. Am J Respir Cell Mol Biol. 2000;22:665–671. - PubMed
-
- Conner GE, Salathe M, Forteza R. Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med. 2002;166:S57–61. - PubMed
-
- Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol. 2003;29:206–212. - PubMed
-
- Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. - PubMed
-
- Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

