Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia
- PMID: 21334467
- PMCID: PMC3096524
- DOI: 10.1016/j.resp.2011.02.008
Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia
Abstract
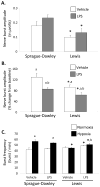
Lipopolysaccharide (LPS) induces inflammatory responses, including microglial activation in the central nervous system. Since LPS impairs certain forms of hippocampal and spinal neuroplasticity, we hypothesized that LPS would impair phrenic long-term facilitation (pLTF) following acute intermittent hypoxia (AIH) in outbred Sprague-Dawley (SD) and inbred Lewis (L) rats. Approximately 3h following a single LPS injection (i.p.), the phrenic response during hypoxic episodes is reduced in both rat strains versus vehicle treated, control rats (SD: 84 ± 7% vs. 128 ± 14% baseline for control, p < 0.05; L: 62 ± 10% vs. 90 ± 9% baseline for control, p < 0.05). At 60 min post-AIH, pLTF is also diminished by LPS in both strains: (SD: 22 ± 5% vs. 73.5 ± 14% baseline for control, p < 0.05; L: 18 ± 15% vs. 56 ± 8% baseline for control, p < 0.05). LPS alone does not affect phrenic burst frequency in either rat strain, suggesting that acute LPS injection has minimal effect on brainstem respiratory rhythm generation. Thus, systemic LPS injections and (presumptive) inflammation impair pLTF, a form of spinal neuroplasticity in respiratory motor control. These results suggest that ongoing infection or inflammation must be carefully considered in studies of respiratory plasticity, or during attempts to harness spinal plasticity as a therapeutic tool in the treatment of respiratory insufficiency, such as spinal cord injury.
Published by Elsevier B.V.
Figures

Similar articles
-
Mild inflammation impairs acute intermittent hypoxia-induced phrenic long-term facilitation by a spinal adenosine-dependent mechanism.J Neurophysiol. 2023 Apr 1;129(4):799-806. doi: 10.1152/jn.00035.2023. Epub 2023 Mar 8. J Neurophysiol. 2023. PMID: 36883762 Free PMC article.
-
Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia.J Appl Physiol (1985). 2013 Apr;114(7):879-87. doi: 10.1152/japplphysiol.01347.2012. Epub 2013 Jan 17. J Appl Physiol (1985). 2013. PMID: 23329821 Free PMC article.
-
Adenosine-dependent phrenic motor facilitation is inflammation resistant.J Neurophysiol. 2017 Feb 1;117(2):836-845. doi: 10.1152/jn.00619.2016. Epub 2016 Dec 7. J Neurophysiol. 2017. PMID: 27927784 Free PMC article.
-
NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation.J Physiol. 2009 May 1;587(Pt 9):1931-42. doi: 10.1113/jphysiol.2008.165597. Epub 2009 Feb 23. J Physiol. 2009. PMID: 19237427 Free PMC article. Review.
-
Hypoxia-induced phrenic long-term facilitation: emergent properties.Ann N Y Acad Sci. 2013 Mar;1279:143-53. doi: 10.1111/nyas.12085. Ann N Y Acad Sci. 2013. PMID: 23531012 Free PMC article. Review.
Cited by
-
Common mechanisms of compensatory respiratory plasticity in spinal neurological disorders.Respir Physiol Neurobiol. 2013 Nov 1;189(2):419-28. doi: 10.1016/j.resp.2013.05.025. Epub 2013 May 28. Respir Physiol Neurobiol. 2013. PMID: 23727226 Free PMC article. Review.
-
Spinal metaplasticity in respiratory motor control.Front Neural Circuits. 2015 Feb 11;9:2. doi: 10.3389/fncir.2015.00002. eCollection 2015. Front Neural Circuits. 2015. PMID: 25717292 Free PMC article. Review.
-
Respiratory function after selective respiratory motor neuron death from intrapleural CTB-saporin injections.Exp Neurol. 2015 May;267:18-29. doi: 10.1016/j.expneurol.2014.11.011. Epub 2014 Dec 2. Exp Neurol. 2015. PMID: 25476493 Free PMC article.
-
Roflumilast, a phosphodiesterase-4 (PDE4) inhibitor, induces respiratory frequency plasticity that is resistant to inflammation in neonatal rat in vitro preparations.Respir Physiol Neurobiol. 2025 Jul;335:104435. doi: 10.1016/j.resp.2025.104435. Epub 2025 Apr 13. Respir Physiol Neurobiol. 2025. PMID: 40228690
-
Systemic inflammation inhibits serotonin receptor 2-induced phrenic motor facilitation upstream from BDNF/TrkB signaling.J Neurophysiol. 2018 Jun 1;119(6):2176-2185. doi: 10.1152/jn.00378.2017. Epub 2018 Mar 7. J Neurophysiol. 2018. PMID: 29513151 Free PMC article.
References
-
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104(2–3):251–60. - PubMed
-
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7(1):48–55. - PubMed
-
- Bessis A, Béchade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55(3):233–238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

