The basement membrane of hair follicle stem cells is a muscle cell niche
- PMID: 21335239
- PMCID: PMC3056115
- DOI: 10.1016/j.cell.2011.01.014
The basement membrane of hair follicle stem cells is a muscle cell niche
Abstract
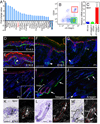
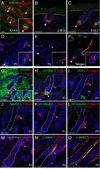
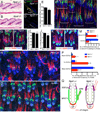
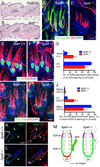
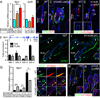
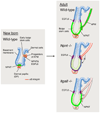
The hair follicle bulge in the epidermis associates with the arrector pili muscle (APM) that is responsible for piloerection ("goosebumps"). We show that stem cells in the bulge deposit nephronectin into the underlying basement membrane, thus regulating the adhesion of mesenchymal cells expressing the nephronectin receptor, α8β1 integrin, to the bulge. Nephronectin induces α8 integrin-positive mesenchymal cells to upregulate smooth muscle markers. In nephronectin knockout mice, fewer arrector pili muscles form in the skin, and they attach to the follicle above the bulge, where there is compensatory upregulation of the nephronectin family member EGFL6. Deletion of α8 integrin also abolishes selective APM anchorage to the bulge. Nephronectin is a Wnt target; epidermal β-catenin activation upregulates epidermal nephronectin and dermal α8 integrin expression. Thus, bulge stem cells, via nephronectin expression, create a smooth muscle cell niche and act as tendon cells for the APM. Our results reveal a functional role for basement membrane heterogeneity in tissue patterning. PAPERCLIP:
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Akiyama M, Dale BA, Sun TT, Holbrook KA. Characterization of hair follicle bulge in human fetal skin: the human fetal bulge is a pool of undifferentiated keratinocytes. J Invest Dermatol. 1995;105:844–850. - PubMed
-
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. - PubMed
-
- Barcaui CB, Pineiro-Maceira J, de Avelar Alchorne MM. Arrector pili muscle: evidence of proximal attachment variant in terminal follicles of the scalp. Br J Dermatol. 2002;146:657–658. - PubMed
-
- Bolcato-Bellemin AL, Lefebvre O, Arnold C, Sorokin L, Miner JH, Kedinger M, Simon-Assmann P. Laminin alpha5 chain is required for intestinal smooth muscle development. Dev Biol. 2003;260:376–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

