The coding of temperature in the Drosophila brain
- PMID: 21335241
- PMCID: PMC3336488
- DOI: 10.1016/j.cell.2011.01.028
The coding of temperature in the Drosophila brain
Abstract
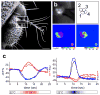
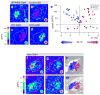
Thermosensation is an indispensable sensory modality. Here, we study temperature coding in Drosophila, and show that temperature is represented by a spatial map of activity in the brain. First, we identify TRP channels that function in the fly antenna to mediate the detection of cold stimuli. Next, we identify the hot-sensing neurons and show that hot and cold antennal receptors project onto distinct, but adjacent glomeruli in the Proximal-Antennal-Protocerebrum (PAP) forming a thermotopic map in the brain. We use two-photon imaging to reveal the functional segregation of hot and cold responses in the PAP, and show that silencing the hot- or cold-sensing neurons produces animals with distinct and discrete deficits in their behavioral responses to thermal stimuli. Together, these results demonstrate that dedicated populations of cells orchestrate behavioral responses to different temperature stimuli, and reveal a labeled-line logic for the coding of temperature information in the brain.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Sensory systems: Mapping thermosensation.Nat Rev Neurosci. 2011 Apr;12(4):187. doi: 10.1038/nrn3017. Epub 2011 Mar 16. Nat Rev Neurosci. 2011. PMID: 21562500 No abstract available.
References
-
- Altner H, Loftus R. Ultrastructure and Function of Insect Thermo- And Hygroreceptors doi:10.1146/annurev.en.30.010185.001421. Annual Review of Entomology. 1985;30:273–295.
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

