S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart
- PMID: 21335477
- PMCID: PMC3094087
- DOI: 10.1152/ajpheart.00946.2010
S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart
Abstract
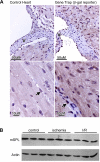
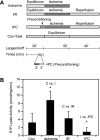
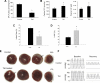
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid that promotes cardiomyocyte survival and contributes to ischemic preconditioning. S1P lyase (SPL) is a stress-activated enzyme responsible for irreversible S1P catabolism. We hypothesized that SPL contributes to oxidative stress by depleting S1P pools available for cardioprotective signaling. Accordingly, we evaluated SPL inhibition as a strategy for reducing cardiac ischemia-reperfusion (I/R) injury. We measured SPL expression and enzyme activity in murine hearts. Basal SPL activity was low in wild-type cardiac tissue but was activated in response to 50 min of ischemia (n = 5, P < 0.01). Hearts of heterozygous SPL knockout mice exhibited reduced SPL activity, elevated S1P levels, smaller infarct size, and increased functional recovery after I/R compared with littermate controls (n = 5, P < 0.01). The small molecule tetrahydroxybutylimidazole (THI) is a Federal Drug Administration-approved food additive that inhibits SPL. When given overnight at 25 mg/l in drinking water, THI raised S1P levels and reduced SPL activity (n = 5, P < 0.01). THI reduced infarct size and enhanced hemodynamic recovery in response to 50 min of ischemia and to 40 min of reperfusion in ex vivo hearts (n = 7, P < .01). These data correlated with an increase in MAP kinase-interacting serine/threonine kinase 1, eukaryotic translation initiation factor 4E, and ribosomal protein S6 phosphorylation levels after I/R, suggesting that SPL inhibition enhances protein translation. Pretreatment with an S1P₁ and S1P₃ receptor antagonist partially reversed the effects of THI. These results reveal, for the first time, that SPL is an ischemia-induced enzyme that can be targeted as a novel strategy for preventing cardiac I/R injury.
Figures



References
-
- Bagdanoff J, Donoviel M, Nouraldeen A, Tarver J, Fu Q, Carlsen M, Jessop T, Zhang H, Hazelwood J, Nguyen H, Baugh S, Gardyan M, Terranova K, Barbosa J, Yan J, Bednarz M, Layek S, Courtney L, Taylor J, Digeorge-Foushee A, Gopinathan S, Bruce D, Smith T, Moran L, O'Neill E, Kramer J, Lai Z, Kimball S, Liu Q, Sun W, Yu S, Swaffield J, Wilson A, Main A, Carson K, Oravecz T, Augeri D. Inhibition of sphingosine-1-phosphate lyase for the treatment of autoimmune disorders. J Med Chem 52: 3941– 3953, 2009 - PubMed
-
- Bandhuvula P, Fyrst H, Saba J. A rapid fluorescent assay for sphingosine-1-phosphate lyase enzyme activity. J Lipid Res 48: 2769– 2778, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

