β2-adrenergic receptor activation prevents rodent dopaminergic neurotoxicity by inhibiting microglia via a novel signaling pathway
- PMID: 21335487
- PMCID: PMC3622942
- DOI: 10.4049/jimmunol.1002449
β2-adrenergic receptor activation prevents rodent dopaminergic neurotoxicity by inhibiting microglia via a novel signaling pathway
Abstract
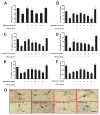
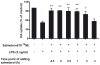
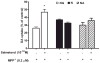
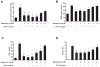
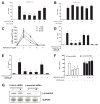
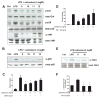
The role of the β2 adrenergic receptor (β2AR) in the regulation of chronic neurodegenerative inflammation within the CNS is poorly understood. The purpose of this study was to determine neuroprotective effects of long-acting β2AR agonists such as salmeterol in rodent models of Parkinson's disease. Results showed salmeterol exerted potent neuroprotection against both LPS and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/1-methyl-4-phenylpyridinium-induced dopaminergic neurotoxicity both in primary neuron-glia cultures (at subnanomolar concentrations) and in mice (1-10 μg/kg/day doses). Further studies demonstrated that salmeterol-mediated neuroprotection is not a direct effect on neurons; instead, it is mediated through the inhibition of LPS-induced microglial activation. Salmeterol significantly inhibited LPS-induced production of microglial proinflammatory neurotoxic mediators, such as TNF-α, superoxide, and NO, as well as the inhibition of TAK1-mediated phosphorylation of MAPK and p65 NF-κB. The anti-inflammatory effects of salmeterol required β2AR expression in microglia but were not mediated through the conventional G protein-coupled receptor/cAMP pathway. Rather, salmeterol failed to induce microglial cAMP production, could not be reversed by either protein kinase A inhibitors or an exchange protein directly activated by cAMP agonist, and was dependent on β-arrestin2 expression. Taken together, our results demonstrate that administration of extremely low doses of salmeterol exhibit potent neuroprotective effects by inhibiting microglial cell activation through a β2AR/β-arrestin2-dependent but cAMP/protein kinase A-independent pathway.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures


References
-
- Koff WC, Fann AV, Dunegan MA, Lachman LB. Catecholamine-induced suppression of interleukin-1 production. Lymphokine Res. 1986;5:239–247. - PubMed
-
- Severn A, Rapson NT, Hunter CA, Liew FY. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992;148:3441–3445. - PubMed
-
- Farmer P, Pugin J. beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am J Physiol Lung Cell Mol Physiol. 2000;279:L675–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

