Structural integrity of {alpha}-helix H12 in translation initiation factor eIF5B is critical for 80S complex stability
- PMID: 21335519
- PMCID: PMC3062179
- DOI: 10.1261/rna.2412511
Structural integrity of {alpha}-helix H12 in translation initiation factor eIF5B is critical for 80S complex stability
Abstract
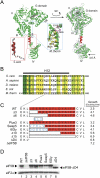
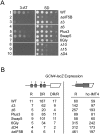
Translation initiation factor eIF5B promotes GTP-dependent ribosomal subunit joining in the final step of the translation initiation pathway. The protein resembles a chalice with the α-helix H12 forming the stem connecting the GTP-binding domain cup to the domain IV base. Helix H12 has been proposed to function as a rigid lever arm governing domain IV movements in response to nucleotide binding and as a molecular ruler fixing the distance between domain IV and the G domain of the factor. To investigate its function, helix H12 was lengthened or shortened by one or two turns. In addition, six consecutive residues in the helix were substituted by Gly to alter the helical rigidity. Whereas the mutations had minimal impacts on the factor's binding to the ribosome and its GTP binding and hydrolysis activities, shortening the helix by six residues impaired the rate of subunit joining in vitro and both this mutation and the Gly substitution mutation lowered the yield of Met-tRNA(i)(Met) bound to 80S complexes formed in the presence of nonhydrolyzable GTP. Thus, these two mutations, which impair yeast cell growth and enhance ribosome leaky scanning in vivo, impair the rate of formation and stability of the 80S product of subunit joining. These data support the notion that helix H12 functions as a ruler connecting the GTPase center of the ribosome to the P site where Met-tRNA(i)(Met) is bound and that helix H12 rigidity is required to stabilize Met-tRNA(i)(Met) binding.
Figures


Similar articles
-
Coupled release of eukaryotic translation initiation factors 5B and 1A from 80S ribosomes following subunit joining.Mol Cell Biol. 2007 Mar;27(6):2384-97. doi: 10.1128/MCB.02254-06. Epub 2007 Jan 22. Mol Cell Biol. 2007. PMID: 17242201 Free PMC article.
-
Interaction between eukaryotic initiation factors 1A and 5B is required for efficient ribosomal subunit joining.J Biol Chem. 2006 Mar 31;281(13):8469-75. doi: 10.1074/jbc.M600210200. Epub 2006 Feb 3. J Biol Chem. 2006. PMID: 16461768
-
The Interaction between the Ribosomal Stalk Proteins and Translation Initiation Factor 5B Promotes Translation Initiation.Mol Cell Biol. 2018 Jul 30;38(16):e00067-18. doi: 10.1128/MCB.00067-18. Print 2018 Aug 15. Mol Cell Biol. 2018. PMID: 29844065 Free PMC article.
-
Universal translation initiation factor IF2/eIF5B.Cold Spring Harb Symp Quant Biol. 2001;66:417-24. doi: 10.1101/sqb.2001.66.417. Cold Spring Harb Symp Quant Biol. 2001. PMID: 12762044 Review. No abstract available.
-
Functional significance and mechanism of eIF5-promoted GTP hydrolysis in eukaryotic translation initiation.Prog Nucleic Acid Res Mol Biol. 2001;70:207-31. doi: 10.1016/s0079-6603(01)70018-9. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11642363 Review.
Cited by
-
Poliovirus switches to an eIF2-independent mode of translation during infection.J Virol. 2011 Sep;85(17):8884-93. doi: 10.1128/JVI.00792-11. Epub 2011 Jun 22. J Virol. 2011. PMID: 21697471 Free PMC article.
-
eIF5B gates the transition from translation initiation to elongation.Nature. 2019 Sep;573(7775):605-608. doi: 10.1038/s41586-019-1561-0. Epub 2019 Sep 18. Nature. 2019. PMID: 31534220 Free PMC article.
-
Structure of the mammalian 80S initiation complex with initiation factor 5B on HCV-IRES RNA.Nat Struct Mol Biol. 2014 Aug;21(8):721-7. doi: 10.1038/nsmb.2859. Epub 2014 Jul 27. Nat Struct Mol Biol. 2014. PMID: 25064512
-
Structural basis for the transition from translation initiation to elongation by an 80S-eIF5B complex.Nat Commun. 2020 Oct 6;11(1):5003. doi: 10.1038/s41467-020-18829-3. Nat Commun. 2020. PMID: 33024099 Free PMC article.
-
Recent Advances in Archaeal Translation Initiation.Front Microbiol. 2020 Sep 18;11:584152. doi: 10.3389/fmicb.2020.584152. eCollection 2020. Front Microbiol. 2020. PMID: 33072057 Free PMC article. Review.
References
-
- Acker MG, Shin BS, Dever TE, Lorsch JR 2006. Interaction between eukaryotic initiation factors 1A and 5B is required for efficient ribosomal subunit joining. J Biol Chem 281: 8469–8475 - PubMed
-
- Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J 2005. The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell 121: 703–712 - PubMed
-
- Choi SK, Lee JH, Zoll WL, Merrick WC, Dever TE 1998. Promotion of Met-tRNAiMet binding to ribosomes by yIF2, a bacterial IF2 homolog in yeast. Science 280: 1757–1760 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
