Cigarette smoke extract suppresses the RIG-I-initiated innate immune response to influenza virus in the human lung
- PMID: 21335520
- PMCID: PMC3119130
- DOI: 10.1152/ajplung.00267.2010
Cigarette smoke extract suppresses the RIG-I-initiated innate immune response to influenza virus in the human lung
Abstract
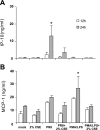
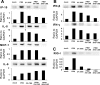
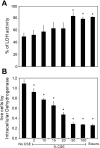
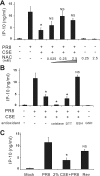
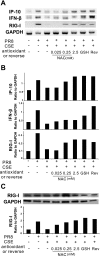
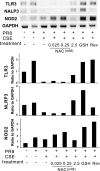
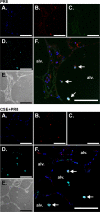
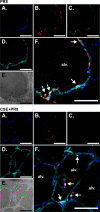
Cigarette smoking is the major cause of chronic obstructive pulmonary disease (COPD) and predisposes subjects to severe respiratory tract infections. Epidemiological studies have shown that cigarette smokers are seven times more likely to contract influenza infection than nonsmokers. The mechanisms underlying this increased susceptibility are poorly characterized. Retinoic acid-inducible gene (RIG)-I is believed to play an important role in the recognition of, and response to, influenza virus and other RNA viruses. Our study focused on how cigarette smoke extract (CSE) alters the influenza-induced proinflammatory response and suppresses host antiviral activity in the human lung using a unique lung organ culture model. We first determined that treatment with 2-20% CSE did not induce cytotoxicity as assessed by LDH release. However, CSE treatment inhibited influenza-induced IFN-inducible protein 10 protein and mRNA expression. Induction of the major antiviral cytokine IFN-β mRNA was also decreased by CSE. CSE also blunted viral-mediated RIG-I mRNA and protein expression. Inhibition of viral-mediated RIG-I induction by CSE was prevented by the antioxidants N-acetyl-cysteine and glutathione. These findings show that CSE suppresses antiviral and innate immune responses in influenza virus-infected human lungs through oxidative inhibition of viral-mediated induction of the pattern recognition receptor RIG-I. This immunosuppressive effect of CSE may play a role in the enhanced susceptibility of smokers to serious influenza infection in the lung.
Figures
Similar articles
-
Influenza A(H1N1)pdm09 virus suppresses RIG-I initiated innate antiviral responses in the human lung.PLoS One. 2012;7(11):e49856. doi: 10.1371/journal.pone.0049856. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185463 Free PMC article.
-
Cigarette smoke attenuates the RIG-I-initiated innate antiviral response to influenza infection in two murine models.Am J Physiol Lung Cell Mol Physiol. 2014 Dec 1;307(11):L848-58. doi: 10.1152/ajplung.00158.2014. Epub 2014 Sep 26. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25260755 Free PMC article.
-
Human primary airway epithelial cells isolated from active smokers have epigenetically impaired antiviral responses.Respir Res. 2016 Sep 7;17(1):111. doi: 10.1186/s12931-016-0428-2. Respir Res. 2016. PMID: 27604339 Free PMC article.
-
The catcher in the RIG-I.Cytokine. 2015 Nov;76(1):38-41. doi: 10.1016/j.cyto.2015.07.002. Epub 2015 Jul 10. Cytokine. 2015. PMID: 26168692 Review.
-
In Vivo and In Vitro Studies of Cigarette Smoke Effects on Innate Responses to Influenza Virus: A Matter of Models?Viruses. 2022 Aug 20;14(8):1824. doi: 10.3390/v14081824. Viruses. 2022. PMID: 36016446 Free PMC article. Review.
Cited by
-
Precision cut lung slices: an integrated ex vivo model for studying lung physiology, pharmacology, disease pathogenesis and drug discovery.Respir Res. 2024 Jun 1;25(1):231. doi: 10.1186/s12931-024-02855-6. Respir Res. 2024. PMID: 38824592 Free PMC article. Review.
-
RIG-I agonist SLR10 promotes macrophage M1 polarization during influenza virus infection.Front Immunol. 2023 Jul 5;14:1177624. doi: 10.3389/fimmu.2023.1177624. eCollection 2023. Front Immunol. 2023. PMID: 37475869 Free PMC article.
-
CYP1B1 knockout enhanced IFN-γ production is required but not sufficient for protection of cigarette smoke-exposed mice against lethal influenza virus infection.Front Immunol. 2025 Jul 4;16:1600025. doi: 10.3389/fimmu.2025.1600025. eCollection 2025. Front Immunol. 2025. PMID: 40688072 Free PMC article.
-
Early IFN-β administration protects cigarette smoke exposed mice against lethal influenza virus infection without increasing lung inflammation.Sci Rep. 2022 Mar 8;12(1):4080. doi: 10.1038/s41598-022-08066-7. Sci Rep. 2022. PMID: 35260752 Free PMC article.
-
Hydrogen peroxide attenuates rhinovirus-induced anti-viral interferon secretion in sinonasal epithelial cells.Front Immunol. 2023 Feb 13;14:1086381. doi: 10.3389/fimmu.2023.1086381. eCollection 2023. Front Immunol. 2023. PMID: 36860857 Free PMC article.
References
-
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20: 319–325, 2004 - PubMed
-
- Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med 164: 2206–2216, 2004 - PubMed
-
- Aronson MD, Weiss ST, Ben RL, Komaroff AL. Association between cigarette smoking and acute respiratory tract illness in young adults. JAMA 248: 181–183, 1982 - PubMed
-
- Baughman RP, Corser BC, Strohofer S, Hendricks D. Spontaneous hydrogen peroxide release from alveolar macrophages of some cigarette smokers. J Lab Clin Med 107: 233–237, 1986 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

