Protein kinase B gene homologue pkbR1 performs one of its roles at first finger stage of Dictyostelium
- PMID: 21335531
- PMCID: PMC3127643
- DOI: 10.1128/EC.00200-10
Protein kinase B gene homologue pkbR1 performs one of its roles at first finger stage of Dictyostelium
Abstract
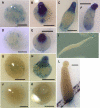
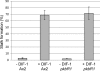
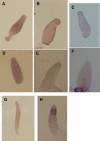
Dictyostelium discoideum has protein kinases AKT/PKBA and PKBR1 that belong to the AGC family of kinases. The protein kinase B-related kinase (PKBR1) has been studied with emphasis on its role in chemotaxis, but its roles in late development remained obscure. The pkbR1 null mutant stays in the first finger stage for about 16 h or longer. Only a few aggregates continue to the migrating slug stage; however, the slugs immediately go back probably to the previous first finger stage and stay there for approximately 37 h. Finally, the mutant fingers diversify into various multicellular bodies. The expression of the pkbR1 finger protein probably is required for development to the slug stage and to express ecmB, which is first observed in migrating slugs. The mutant also showed no ST-lacZ expression, which is of the earliest step in differentiation to one of the stalk cell subtypes. The pkbR1 null mutant forms a small number of aberrant fruiting bodies, but in the presence of 10% of wild-type amoebae the mutant preferentially forms viable spores, driving the wild type to form nonviable stalk cells. These results suggest that the mutant has defects in a system that changes the physiological dynamics in the prestalk cell region of a finger. We suggest that the arrest of its development is due to the loss of the second wave of expression of a protein kinase A catalytic subunit gene (pkaC) only in the prestalk region of the pkbR1 null mutant.
Figures



Similar articles
-
Evidence that the Dictyostelium Dd-STATa protein is a repressor that regulates commitment to stalk cell differentiation and is also required for efficient chemotaxis.Development. 1999 Aug;126(15):3391-405. doi: 10.1242/dev.126.15.3391. Development. 1999. PMID: 10393118
-
PP2A/B56 and GSK3/Ras suppress PKB activity during Dictyostelium chemotaxis.Mol Biol Cell. 2015 Dec 1;26(24):4347-57. doi: 10.1091/mbc.E14-06-1130. Epub 2015 Sep 30. Mol Biol Cell. 2015. PMID: 26424797 Free PMC article.
-
Chemotactic activation of Dictyostelium AGC-family kinases AKT and PKBR1 requires separate but coordinated functions of PDK1 and TORC2.J Cell Sci. 2010 Mar 15;123(Pt 6):983-92. doi: 10.1242/jcs.064022. J Cell Sci. 2010. PMID: 20200230 Free PMC article.
-
Developmental decisions in Dictyostelium discoideum.Microbiol Rev. 1994 Sep;58(3):330-51. doi: 10.1128/mr.58.3.330-351.1994. Microbiol Rev. 1994. PMID: 7968918 Free PMC article. Review.
-
The control of chemotactic cell movement during Dictyostelium morphogenesis.Philos Trans R Soc Lond B Biol Sci. 2000 Jul 29;355(1399):983-91. doi: 10.1098/rstb.2000.0634. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11128992 Free PMC article. Review.
References
-
- Abe K., Yanagisawa K. 1983. A new class of rapidly developing mutants in Dictyostelium discoideum: implications for cyclic AMP metabolism and cell differentiation. Dev. Biol. 95:200–210 - PubMed
-
- Anjard C., Loomis W. F. 2006. GABA induces terminal differentiation of Dictyostelium through a GABAB receptor. Development 133:2253–2261 - PubMed
-
- Berks M., Kay R. R. 1988. Cyclic AMP is an inhibitor of stalk cell differentiation in Dictyostelium discoideum. Dev. Biol. 126:108–114 - PubMed
-
- Ceccarelli A., Mahbubani H., Williams J. G. 1991. Positively and negatively acting signals regulating stalk cell and anterior-like cell differentiation in Dictyostelium. Cell 65:983–989 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

