Effects of ultraviolet-A and riboflavin on the interaction of collagen and proteoglycans during corneal cross-linking
- PMID: 21335557
- PMCID: PMC3075647
- DOI: 10.1074/jbc.M110.169813
Effects of ultraviolet-A and riboflavin on the interaction of collagen and proteoglycans during corneal cross-linking
Abstract
Corneal cross-linking using riboflavin and ultraviolet-A (RFUVA) is a clinical treatment targeting the stroma in progressive keratoconus. The stroma contains keratocan, lumican, mimecan, and decorin, core proteins of major proteoglycans (PGs) that bind collagen fibrils, playing important roles in stromal transparency. Here, a model reaction system using purified, non-glycosylated PG core proteins in solution in vitro has been compared with reactions inside an intact cornea, ex vivo, revealing effects of RFUVA on interactions between PGs and collagen cross-linking. Irradiation with UVA and riboflavin cross-links collagen α and β chains into larger polymers. In addition, RFUVA cross-links PG core proteins, forming higher molecular weight polymers. When collagen type I is mixed with individual purified, non-glycosylated PG core proteins in solution in vitro and subjected to RFUVA, both keratocan and lumican strongly inhibit collagen cross-linking. However, mimecan and decorin do not inhibit but instead form cross-links with collagen, forming new high molecular weight polymers. In contrast, corneal glycosaminoglycans, keratan sulfate and chondroitin sulfate, in isolation from their core proteins, are not cross-linked by RFUVA and do not form cross-links with collagen. Significantly, when RFUVA is conducted on intact corneas ex vivo, both keratocan and lumican, in their natively glycosylated form, do form cross-links with collagen. Thus, RFUVA causes cross-linking of collagen molecules among themselves and PG core proteins among themselves, together with limited linkages between collagen and keratocan, lumican, mimecan, and decorin. RFUVA as a diagnostic tool reveals that keratocan and lumican core proteins interact with collagen very differently than do mimecan and decorin.
Figures
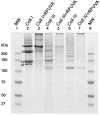

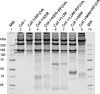
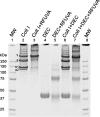
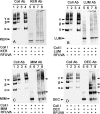




Similar articles
-
Resistance of corneal RFUVA–cross-linked collagens and small leucine-rich proteoglycans to degradation by matrix metalloproteinases.Invest Ophthalmol Vis Sci. 2013 Feb 5;54(2):1014-25. doi: 10.1167/iovs.12-11277. Invest Ophthalmol Vis Sci. 2013. PMID: 23322569 Free PMC article.
-
Expression of the keratan sulfate proteoglycans lumican, keratocan and osteoglycin/mimecan during chick corneal development.Exp Eye Res. 2000 Mar;70(3):349-62. doi: 10.1006/exer.1999.0789. Exp Eye Res. 2000. PMID: 10712821
-
Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA).Invest Ophthalmol Vis Sci. 2010 Jan;51(1):129-38. doi: 10.1167/iovs.09-3738. Epub 2009 Jul 30. Invest Ophthalmol Vis Sci. 2010. PMID: 19643975 Free PMC article.
-
Roles of lumican and keratocan on corneal transparency.Glycoconj J. 2002 May-Jun;19(4-5):275-85. doi: 10.1023/A:1025396316169. Glycoconj J. 2002. PMID: 12975606 Review.
-
The molecular basis of corneal transparency.Exp Eye Res. 2010 Sep;91(3):326-35. doi: 10.1016/j.exer.2010.06.021. Epub 2010 Jul 3. Exp Eye Res. 2010. PMID: 20599432 Free PMC article. Review.
Cited by
-
Detecting early changes in choroidal vascularity and thickness using optical coherence tomography in patients with corneal crosslinking for keratoconus.Int J Ophthalmol. 2024 Jul 18;17(7):1267-1272. doi: 10.18240/ijo.2024.07.11. eCollection 2024. Int J Ophthalmol. 2024. PMID: 39026917 Free PMC article.
-
Mapping the differential distribution of proteoglycan core proteins in the adult human retina, choroid, and sclera.Invest Ophthalmol Vis Sci. 2012 Nov 7;53(12):7528-38. doi: 10.1167/iovs.12-10797. Invest Ophthalmol Vis Sci. 2012. PMID: 23074202 Free PMC article.
-
Extracellular Matrix Stiffness and TGFβ2 Regulate YAP/TAZ Activity in Human Trabecular Meshwork Cells.Front Cell Dev Biol. 2022 Mar 1;10:844342. doi: 10.3389/fcell.2022.844342. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35300422 Free PMC article.
-
Long term results of a prospective randomized bilateral eye comparison trial of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross linking for progressive keratoconus.Clin Ophthalmol. 2012;6:97-101. doi: 10.2147/OPTH.S27170. Epub 2012 Jan 11. Clin Ophthalmol. 2012. PMID: 22275813 Free PMC article.
-
A randomized clinical trial assessing theranostic-guided corneal cross-linking for treating keratoconus: the ARGO protocol.Int Ophthalmol. 2023 Jul;43(7):2315-2328. doi: 10.1007/s10792-022-02628-4. Epub 2022 Dec 31. Int Ophthalmol. 2023. PMID: 36587174 Free PMC article.
References
-
- Beuerman R. W., Pedroza L. (1996) Microsc. Res. Tech. 33, 320–335 - PubMed
-
- Marfurt C. F., Cox J., Deek S., Dvorscak L. (2010) Exp. Eye Res. 90, 478–492 - PubMed
-
- Al-Aqaba M. A., Fares U., Suleman H., Lowe J., Dua H. S. (2010) Br. J. Ophthalmol. 94, 784–789 - PubMed
-
- Müller L. J., Pels L., Vrensen G. F. (1996) Invest. Ophthalmol. Vis. Sci. 37, 476–488 - PubMed
-
- Worthington C. R. (1984) Q. Rev. Biophys. 17, 423–451 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

