Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts
- PMID: 21335558
- PMCID: PMC3075638
- DOI: 10.1074/jbc.M110.143693
Collagen I induces discoidin domain receptor (DDR) 1 expression through DDR2 and a JAK2-ERK1/2-mediated mechanism in primary human lung fibroblasts
Abstract
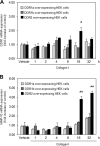
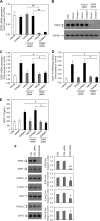
Discoidin domain receptors (DDRs) DDR1 and DDR2 are receptor tyrosine kinases with the unique ability among receptor tyrosine kinases to respond to collagen. Several signaling molecules have been implicated in DDR signaling, including Shp-2, Src, and MAPK pathways, but a detailed understanding of these pathways and their transcriptional targets is still lacking. Similarly, the regulation of the expression of DDRs is poorly characterized with only a few inflammatory mediators, such as lipopolysaccharide and interleukin-1β identified as playing a role in DDR1 expression. DDRs have been reported to induce the expression of various genes including matrix metalloproteinases and bone morphogenetic proteins, but the regulatory mechanisms underlying DDR-induced gene expression remain to be determined. The aim of the present work was to elucidate the molecular mechanisms implicated in the expression of DDRs and to identify DDR-induced signaling pathways and target genes. Our data show that collagen I induces the expression of DDR1 in a dose- and time-dependent manner in primary human lung fibroblasts. Furthermore, activation of DDR2, JAK2, and ERK1/2 MAPK signaling pathways was essential for collagen I-induced DDR1 and matrix metalloproteinase 10 expression. Finally, inhibition of the ERK1/2 pathway abrogated DDR1 expression by blocking the recruitment of the transcription factor polyoma enhancer A-binding protein 3 to the DDR1 promoter. Our data provide new insights into the molecular mechanisms of collagen I-induced DDR1 expression and demonstrate an important role for ERK1/2 activation and the recruitment of polyoma enhancer-A binding protein 3 to the DDR1 promoter.
Figures








References
-
- Alves F., Vogel W., Mossie K., Millauer B., Höfler H., Ullrich A. (1995) Oncogene 10, 609–618 - PubMed
-
- Vogel W., Gish G. D., Alves F., Pawson T. (1997) Mol. Cell 1, 13–23 - PubMed
-
- Shrivastava A., Radziejewski C., Campbell E., Kovac L., McGlynn M., Ryan T. E., Davis S., Goldfarb M. P., Glass D. J., Lemke G., Yancopoulos G. D. (1997) Mol. Cell 1, 25–34 - PubMed
-
- Vogel W. F., Abdulhussein R., Ford C. E. (2006) Cell Signal. 18, 1108–1116 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

