ERG gene rearrangements are common in prostatic small cell carcinomas
- PMID: 21336263
- PMCID: PMC3484363
- DOI: 10.1038/modpathol.2011.7
ERG gene rearrangements are common in prostatic small cell carcinomas
Abstract
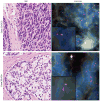
Small cell carcinoma of the prostate is a rare subtype with an aggressive clinical course. Despite the frequent occurrence of ERG gene rearrangements in acinar carcinoma, the incidence of these rearrangements in prostatic small cell carcinoma is unclear. In addition, molecular markers to distinguish prostatic small cell carcinomas from lung and bladder small cell carcinomas may be clinically useful. We examined the occurrence of ERG gene rearrangements by fluorescence in situ hybridization in prostatic, bladder and lung small cell carcinomas. We also examined the expression of ERG, androgen receptor (AR) and NKX3-1 by immunohistochemistry in prostatic cases. Overall, 45% (10/22) of prostatic small cell carcinoma cases harbored ERG rearrangements, whereas no cases of bladder or lung small cell carcinomas showed ERG rearrangement (0/12 and 0/13, respectively). Of prostatic small cell carcinoma cases, 80% (8/10) showed ERG deletion and 20% (2/10) showed ERG translocation. In 83% (5/6) of prostatic small cell carcinoma cases in which a concurrent conventional prostatic acinar carcinoma component was available for analysis, there was concordance for the presence/absence of ERG gene rearrangement between the different subtypes. ERG, AR and NKX3-1 protein expression was detected in a minority of prostatic small cell carcinoma cases (23, 27 and 18%, respectively), while these markers were positive in the majority of concurrent acinar carcinoma cases (66, 83 and 83%, respectively). The presence of ERG rearrangements in nearly half of the prostatic small cell carcinomas is a similar rate of rearrangement to that found in prostatic acinar carcinomas. Furthermore, the high concordance rate of ERG rearrangement between the small cell and acinar components in a given patient supports a common origin for these two subtypes of prostate cancer. Finally, the absence of ERG rearrangement in bladder or lung small cell carcinomas highlights the utility of detecting ERG rearrangement in small cell carcinomas of unknown primary for establishing prostatic origin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Epstein JI, Netto GN. Biopsy Interpretation of the Prostate. 2. Lippincott, Williams & Wilkins; Philadelphia, PA: 2008.
-
- Palmgren JS, Karavadia SS, Wakefield MR. Unusual and underappreciated: small cell carcinoma of the prostate. Semin Oncol. 2007;34:22–29. - PubMed
-
- Helpap B, Kollermann J. Undifferentiated carcinoma of the prostate with small cell features: immunohistochemical subtyping and reflections on histogenesis. Virchows Arch. 1999;434:385–391. - PubMed
-
- Yao JL, Madeb R, Bourne P, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol. 2006;30:705–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

