Controlling protein translocation through nanopores with bio-inspired fluid walls
- PMID: 21336266
- PMCID: PMC3071889
- DOI: 10.1038/nnano.2011.12
Controlling protein translocation through nanopores with bio-inspired fluid walls
Abstract
Synthetic nanopores have been used to study individual biomolecules in high throughput, but their performance as sensors does not match that of biological ion channels. Challenges include control of nanopore diameters and surface chemistry, modification of the translocation times of single-molecule analytes through nanopores, and prevention of non-specific interactions with pore walls. Here, inspired by the olfactory sensilla of insect antennae, we show that coating nanopores with a fluid lipid bilayer tailors their surface chemistry and allows fine-tuning and dynamic variation of pore diameters in subnanometre increments. Incorporation of mobile ligands in the lipid bilayer conferred specificity and slowed the translocation of targeted proteins sufficiently to time-resolve translocation events of individual proteins. Lipid coatings also prevented pores from clogging, eliminated non-specific binding and enabled the translocation of amyloid-beta (Aβ) oligomers and fibrils. Through combined analysis of their translocation time, volume, charge, shape and ligand affinity, different proteins were identified.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



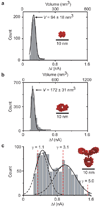
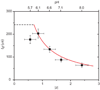

Comment in
-
Nanobiotechnology: A new look for nanopore sensing.Nat Nanotechnol. 2011 Apr;6(4):195-6. doi: 10.1038/nnano.2011.52. Nat Nanotechnol. 2011. PMID: 21468105
References
-
- Sexton LT, et al. Resistive-pulse studies of proteins and protein/antibody complexes using a conical nanotube sensor. J. Am. Chem. Soc. 2007;129:13144–13152. - PubMed
-
- Movileanu L, Howorka S, Braha O, Bayley H. Detecting protein analytes that modulate transmembrane movement of a polymer chain within a single protein pore. Nature Biotech. 2000;18:1091–1095. - PubMed
-
- Howorka S, Siwy Z. Nanopore analytics: sensing of single molecules. Chem. Soc. Rev. 2009;38:2360–2384. - PubMed
-
- Siwy Z, et al. Protein biosensors based on biofunctionalized conical gold nanotubes. J. Am. Chem. Soc. 2005;127:5000–5001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

