Mapping of INS promoter interactions reveals its role in long-range regulation of SYT8 transcription
- PMID: 21336277
- PMCID: PMC6402586
- DOI: 10.1038/nsmb.1993
Mapping of INS promoter interactions reveals its role in long-range regulation of SYT8 transcription
Abstract
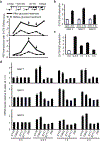
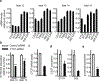
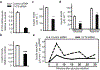
Insulin (INS) synthesis and secretion from pancreatic β-cells are tightly regulated; their deregulation causes diabetes. Here we map INS-associated loci in human pancreatic islets by 4C and 3C techniques and show that the INS gene physically interacts with the SYT8 gene, located over 300 kb away. This interaction is elevated by glucose and accompanied by increases in SYT8 expression. Inactivation of the INS promoter by promoter-targeting siRNA reduces SYT8 gene expression. SYT8-INS interaction and SYT8 transcription are attenuated by CTCF depletion. Furthermore, SYT8 knockdown decreases insulin secretion in islets. These results reveal a nonredundant role for SYT8 in insulin secretion and indicate that the INS promoter acts from a distance to stimulate SYT8 transcription. This suggests a function for the INS promoter in coordinating insulin transcription and secretion through long-range regulation of SYT8 expression in human islets.
Figures

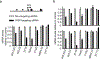
References
-
- Apostolou E & Thanos D Virus Infection Induces NF-kB-dependent interchromosomal associations mediating monoallelic IFN-β gene expression. Cell 134, 85–96 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

