How APC/C-Cdc20 changes its substrate specificity in mitosis
- PMID: 21336306
- PMCID: PMC3059483
- DOI: 10.1038/ncb2165
How APC/C-Cdc20 changes its substrate specificity in mitosis
Erratum in
- Nat Cell Biol. 2011 May;13(5):633
Abstract
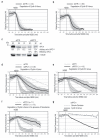
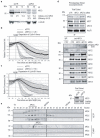
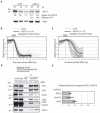
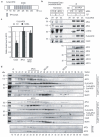
Progress through mitosis requires that the right protein be degraded at the right time. One ubiquitin ligase, the anaphase-promoting complex or cyclosome (APC/C) targets most of the crucial mitotic regulators by changing its substrate specificity throughout mitosis. The spindle assembly checkpoint (SAC) acts on the APC/C co-activator, Cdc20 (cell division cycle 20), to block the degradation of metaphase substrates (for example, cyclin B1 and securin), but not others (for example, cyclin A). How this is achieved is unclear. Here we show that Cdc20 binds to different sites on the APC/C depending on the SAC. Cdc20 requires APC3 and APC8 to bind and activate the APC/C when the SAC is satisfied, but requires only APC8 to bind the APC/C when the SAC is active. Moreover, APC10 is crucial for the destruction of cyclin B1 and securin, but not cyclin A. We conclude that the SAC causes Cdc20 to bind to different sites on the APC/C and this alters APC/C substrate specificity.
© 2011 Macmillan Publishers Limited. All rights reserved.
Figures



References
-
- Pines J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 2006;16:55–63. - PubMed
-
- Peters JM. The anaphase promoting complex-cyclosome: a machine designed to destroy. Nature reviews. 2006;7:644–656. - PubMed
-
- Gmachl M, Gieffers C, Podtelejnikov AV, Mann M, Peters JM. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8973–8978. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

