p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase
- PMID: 21336310
- PMCID: PMC3110666
- DOI: 10.1038/ncb2172
p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase
Abstract
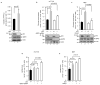
Cancer cells consume large quantities of glucose and primarily use glycolysis for ATP production, even in the presence of adequate oxygen. This metabolic signature (aerobic glycolysis or the Warburg effect) enables cancer cells to direct glucose to biosynthesis, supporting their rapid growth and proliferation. However, both causes of the Warburg effect and its connection to biosynthesis are not well understood. Here we show that the tumour suppressor p53, the most frequently mutated gene in human tumours, inhibits the pentose phosphate pathway (PPP). Through the PPP, p53 suppresses glucose consumption, NADPH production and biosynthesis. The p53 protein binds to glucose-6-phosphate dehydrogenase (G6PD), the first and rate-limiting enzyme of the PPP, and prevents the formation of the active dimer. Tumour-associated p53 mutants lack the G6PD-inhibitory activity. Therefore, enhanced PPP glucose flux due to p53 inactivation may increase glucose consumption and direct glucose towards biosynthesis in tumour cells.
© 2011 Macmillan Publishers Limited. All rights reserved.
Conflict of interest statement
Figures
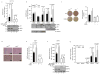



Comment in
-
p53 guards the metabolic pathway less travelled.Nat Cell Biol. 2011 Mar;13(3):195-7. doi: 10.1038/ncb2177. Epub 2011 Feb 20. Nat Cell Biol. 2011. PMID: 21336302
References
-
- Warburg O, Posener K, Negelein E. Ueber den Stoffwechsel der Tumoren. Biochem Z. 1924;152:319–344.
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. - PubMed
-
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. 2007:577–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

