Signal quality of single dose gadobenate dimeglumine pulmonary MRA examinations exceeds quality of MRA performed with double dose gadopentetate dimeglumine
- PMID: 21337023
- PMCID: PMC3141086
- DOI: 10.1007/s10554-011-9821-6
Signal quality of single dose gadobenate dimeglumine pulmonary MRA examinations exceeds quality of MRA performed with double dose gadopentetate dimeglumine
Abstract
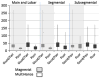
During a recent multi-center trial assessing gadolinium (Gd)-enhanced magnetic resonance angiography (MRA) for diagnosis of acute pulmonary embolism (PE), the Food and Drug Administration announced a risk of nephrogenic sclerosing fibrosis in patients with renal insufficiency who had received intravenous Gd-based MR contrast agents. Although no patients in this trial had renal insufficiency, in cautious response to this announcement, the trial protocol was changed from an intravenous administration of 0.2 mmol/Kg of a conventional Gd-based MR contrast agent to 0.1 mmol/Kg of gadobenate dimeglumine. The study described herein compares the signal quality of pulmonary MRA performed with double dose conventional agent to single dose gadobenate dimeglumine. This study is a retrospective analysis of data from a prospective, multicenter study in men and women ≥18 years with documented presence or absence of PE. The study was approved by the Institutional Review Board at all participating centers, and all patients provided written indication of informed consent. We performed both objective and subjective analysis of pulmonary artery image quality. Signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) in the main pulmonary artery were assessed in single and double dose protocols and compared. SNR and CNR of the main PA were correlated with subjective quality assessment of main/lobar, segmental and subsegmental pulmonary arteries. Although there were individual outliers, both SNR (P = 0.01) and CNR (P = 0.008) were higher in all quartiles for examinations using gadobenate dimeglumine than with gadopentetate dimeglumine. Subjective quality of vascular signal intensity at each vessel order was significantly better for gadobenate dimeglumine (P < 0.0001), and correlated well with SNR and CNR at each order (<0.001). Because of agent high relaxivity, a single dose of gadobenate dimeglumine provides better pulmonary MRA signal quality than double dose of a conventional Gd-based MR contrast agent.
Conflict of interest statement
Figures


Comment in
-
MR Pulmonary angiography: assessment of PIOPED III data.Int J Cardiovasc Imaging. 2012 Feb;28(2):313-5. doi: 10.1007/s10554-011-9835-0. Epub 2011 Feb 24. Int J Cardiovasc Imaging. 2012. PMID: 21347591 No abstract available.
Similar articles
-
Multicenter, intraindividual comparison of single-dose gadobenate dimeglumine and double-dose gadopentetate dimeglumine for MR angiography of the supra-aortic arteries (the Supra-Aortic VALUE study).AJNR Am J Neuroradiol. 2013 Apr;34(4):847-54. doi: 10.3174/ajnr.A3298. Epub 2012 Oct 4. AJNR Am J Neuroradiol. 2013. PMID: 23042922 Free PMC article. Clinical Trial.
-
Contrast-enhanced MR Angiography of the renal arteries: blinded multicenter crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine.Radiology. 2005 Feb;234(2):399-408. doi: 10.1148/radiol.2342040023. Epub 2004 Dec 22. Radiology. 2005. PMID: 15616119 Clinical Trial.
-
Dynamic and static magnetic resonance angiography of the supra-aortic vessels at 3.0 T: intraindividual comparison of gadobutrol, gadobenate dimeglumine, and gadoterate meglumine at equimolar dose.Invest Radiol. 2013 Mar;48(3):121-8. doi: 10.1097/RLI.0b013e31827752b4. Invest Radiol. 2013. PMID: 23211552 Free PMC article. Clinical Trial.
-
Multicenter, intra-individual comparison of single dose gadobenate dimeglumine and double dose gadopentetate dimeglumine for MR angiography of the peripheral arteries (the Peripheral VALUE Study).J Magn Reson Imaging. 2013 Oct;38(4):926-37. doi: 10.1002/jmri.24040. Epub 2013 Jan 31. J Magn Reson Imaging. 2013. PMID: 23371919 Clinical Trial.
-
Gadobenate dimeglumine (MultiHance) for magnetic resonance angiography: review of the literature.Eur Radiol. 2003 Nov;13 Suppl 3:N19-27. doi: 10.1007/s00330-003-0003-3. Eur Radiol. 2003. PMID: 15015877 Review.
Cited by
-
Multicenter, intraindividual comparison of single-dose gadobenate dimeglumine and double-dose gadopentetate dimeglumine for MR angiography of the supra-aortic arteries (the Supra-Aortic VALUE study).AJNR Am J Neuroradiol. 2013 Apr;34(4):847-54. doi: 10.3174/ajnr.A3298. Epub 2012 Oct 4. AJNR Am J Neuroradiol. 2013. PMID: 23042922 Free PMC article. Clinical Trial.
-
MR Pulmonary angiography: assessment of PIOPED III data.Int J Cardiovasc Imaging. 2012 Feb;28(2):313-5. doi: 10.1007/s10554-011-9835-0. Epub 2011 Feb 24. Int J Cardiovasc Imaging. 2012. PMID: 21347591 No abstract available.
-
Factors in the technical quality of gadolinium enhanced magnetic resonance angiography for pulmonary embolism in PIOPED III.Int J Cardiovasc Imaging. 2012 Feb;28(2):303-12. doi: 10.1007/s10554-011-9820-7. Epub 2011 Feb 24. Int J Cardiovasc Imaging. 2012. PMID: 21347594 Free PMC article.
-
Low-dose gadobenate dimeglumine-enhanced MRI of the kidney for the differential diagnosis of localized renal lesions.Radiol Med. 2015 Dec;120(12):1100-11. doi: 10.1007/s11547-015-0548-7. Epub 2015 Jun 19. Radiol Med. 2015. PMID: 26088468 Free PMC article.
-
Breast lesion detection and characterization with contrast-enhanced magnetic resonance imaging: Prospective randomized intraindividual comparison of gadoterate meglumine (0.15 mmol/kg) and gadobenate dimeglumine (0.075 mmol/kg) at 3T.J Magn Reson Imaging. 2019 Apr;49(4):1157-1165. doi: 10.1002/jmri.26335. Epub 2018 Dec 15. J Magn Reson Imaging. 2019. PMID: 30552829 Free PMC article. Clinical Trial.
References
-
- Stein PD, Chenevert TL, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Jablonski KA, Leeper KV, Naidich DP, Sak DJ, Sostman HD, Tapson VF, Weg JG, Woodard PK. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multi-center prospective study (PIOPED III) Annals Int Med. 2010;152(7):434–443. - PMC - PubMed
-
- US Food and Drug Administration. Information on gadolinium-containing contrast agents. [24 Sep 2010];2010 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa....
-
- Cowper SE. International Center for Nephrogenic Fibrosing Dermopathy Research (ICNFDR) 2009 http://www.pathmax.com/dermweb/ last updated 25 Oct 2009.
-
- Prince MR, Arnoldus C, Frisoli JK. Nephrotoxicity of high-dose gadolinium compared with iodinated contrast. J Magn Reson Imaging. 1996;6(1):162–166. - PubMed
-
- Ledneva E, Karie S, Launay-Vacher V, Janus N, Deray G. Renal safety of gadolinium-based contrast media in patients with chronic renal insufficiency. Radiology. 2009;250(3):618–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL077154/HL/NHLBI NIH HHS/United States
- U01 HL077149/HL/NHLBI NIH HHS/United States
- HL177150/HL/NHLBI NIH HHS/United States
- HL077153/HL/NHLBI NIH HHS/United States
- U01 HL077154/HL/NHLBI NIH HHS/United States
- U01 HL077153/HL/NHLBI NIH HHS/United States
- HL077358/HL/NHLBI NIH HHS/United States
- HL077151/HL/NHLBI NIH HHS/United States
- HL081593/HL/NHLBI NIH HHS/United States
- HL077155/HL/NHLBI NIH HHS/United States
- U01 HL081593/HL/NHLBI NIH HHS/United States
- U01 HL077151/HL/NHLBI NIH HHS/United States
- HL081594/HL/NHLBI NIH HHS/United States
- U01 HL077358/HL/NHLBI NIH HHS/United States
- HL077149/HL/NHLBI NIH HHS/United States
- U01 HL077155/HL/NHLBI NIH HHS/United States
- U01 HL081594/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

