MESD is essential for apical localization of megalin/LRP2 in the visceral endoderm
- PMID: 21337463
- PMCID: PMC3069535
- DOI: 10.1002/dvdy.22477
MESD is essential for apical localization of megalin/LRP2 in the visceral endoderm
Abstract
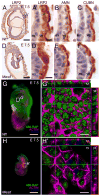
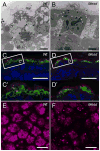
Deletion of the Mesd gene region blocks gastrulation and mesoderm differentiation in mice. MESD is a chaperone for the Wnt co-receptors: low-density lipoprotein receptor-related protein (LRP) 5 and 6 (LRP5/6). We hypothesized that loss of Wnt signaling is responsible for the polarity defects observed in Mesd-deficient embryos. However, because the Mesd-deficient embryo is considerably smaller than Lrp5/6 or Wnt3 mutants, we predicted that MESD function extends more broadly to the LRP family of receptors. Consistent with this prediction, we demonstrated that MESD function in vitro was essential for maturation of the β-propeller/EGF domain common to LRPs. To begin to understand the role of MESD in LRP maturation in vivo, we generated a targeted Mesd knockout and verified that loss of Mesd blocks WNT signaling in vivo. Mesd mutants continue to express the pluripotency markers Oct4, Nanog, and Sox2, suggesting that Wnt signaling is essential for differentiation of the epiblast. Moreover, we demonstrated that MESD was essential for the apical localization of the related LRP2 (Megalin/MEG) in the visceral endoderm, resulting in impaired endocytic function. Combined, our results provide evidence that MESD functions as a general LRP chaperone and suggest that the Mesd phenotype results from both signaling and endocytic defects resulting from misfolding of multiple LRP receptors.
Copyright © 2010 Wiley-Liss, Inc.
Figures





References
-
- Andersen OM, Christensen LL, Christensen PA, Sorensen ES, Jacobsen C, Moestrup SK, Etzerodt M, Thogersen HC. Identification of the minimal functional unit in the low density lipoprotein receptor-related protein for binding the receptor-associated protein (RAP). A conserved acidic residue in the complement-type repeats is important for recognition of RAP. J Biol Chem. 2000;275:21017–21024. - PubMed
-
- Baron MH. Early patterning of the mouse embryo: implications for hematopoietic commitment and differentiation. Exp Hematol. 2005;33:1015–1020. - PubMed
-
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

