Dynamic smad-mediated BMP signaling revealed through transgenic zebrafish
- PMID: 21337469
- PMCID: PMC3072245
- DOI: 10.1002/dvdy.22567
Dynamic smad-mediated BMP signaling revealed through transgenic zebrafish
Abstract
Bone morphogenic protein (BMP) signaling is fundamental to development, injury response, and homeostasis. We have developed transgenic zebrafish that report Smad-mediated BMP signaling in embryos and adults. These lines express either enhanced green fluorescent protein (eGFP), destabilized eGFP, or destabilized Kusabira Orange 2 (KO2) under the well-characterized BMP Response Element (BRE). These fluorescent proteins were found to be expressed dynamically in regions of known BMP signaling including the developing tail bud, hematopoietic lineage, dorsal eye, brain structures, heart, jaw, fins, and somites, as well as other tissues. Responsiveness to changes in BMP signaling was confirmed by observing fluorescence after activation in an hsp70:bmp2b transgenic background or by inhibition in an hsp70:nog3 background. We further demonstrated faithful reportage by the BRE transgenic lines following chemical repression of BMP signaling using an inhibitor of BMP receptor activity, dorsomorphin. Overall, these lines will serve as valuable tools to explore the mechanisms and regulation of BMP signal during embryogenesis, in tissue maintenance, and during disease.
Copyright © 2011 Wiley-Liss, Inc.
Figures







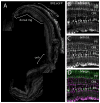
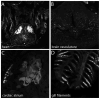


References
-
- Banas MC, Parks WT, Hudkins KL, Banas B, Holdren M, Iyoda M, Wietecha TA, Kowalewska J, Liu G, Alpers CE. Localization of TGF-beta signaling intermediates Smad2, 3, 4, and 7 in developing and mature human and mouse kidney. J Histochem Cytochem. 2007;55(3):275–85. - PubMed
-
- Bauer H, Lele Z, Rauch GJ, Geisler R, Hammerschmidt M. The type I serine/threonine kinase receptor Alk8/Lost-a-fin is required for Bmp2b/7 signal transduction during dorsoventral patterning of the zebrafish embryo. Development. 2001;128(6):849–58. - PubMed
-
- Blitz IL, Cho KW. Finding partners: how BMPs select their targets. Dev Dyn. 2009;238(6):1321–31. - PubMed
-
- Connors SA, Trout J, Ekker M, Mullins MC. The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development. 1999;126(14):3119–30. - PubMed
-
- Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF, Lendahl U. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130(24):6089–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

