Residue-specific fluorescent probes of α-synuclein: detection of early events at the N- and C-termini during fibril assembly
- PMID: 21338068
- PMCID: PMC3074234
- DOI: 10.1021/bi2000824
Residue-specific fluorescent probes of α-synuclein: detection of early events at the N- and C-termini during fibril assembly
Abstract
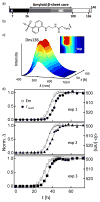
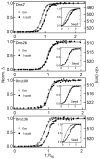
In the Parkinson's disease-associated state, α-synuclein undergoes large conformational changes, forming ordered, β-sheet-containing fibrils. To unravel the role of specific residues during the fibril assembly process, we prepared single-Cys mutants in the disordered (G7C and Y136C) and proximal (V26C and L100C) fibril core sites and derivatized them with environmentally sensitive dansyl (Dns) fluorophores. Dns fluorescence exhibits residue specificity in spectroscopic properties as well as kinetic behavior; early kinetic events were revealed by probes located at positions 7 and 136 compared to those at positions 26 and 100.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

