Electrons, photons, and force: quantitative single-molecule measurements from physics to biology
- PMID: 21338175
- PMCID: PMC3043607
- DOI: 10.1021/nn103298x
Electrons, photons, and force: quantitative single-molecule measurements from physics to biology
Abstract
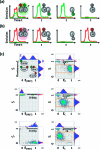
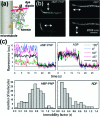
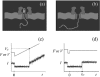
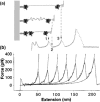
Single-molecule measurement techniques have illuminated unprecedented details of chemical behavior, including observations of the motion of a single molecule on a surface, and even the vibration of a single bond within a molecule. Such measurements are critical to our understanding of entities ranging from single atoms to the most complex protein assemblies. We provide an overview of the strikingly diverse classes of measurements that can be used to quantify single-molecule properties, including those of single macromolecules and single molecular assemblies, and discuss the quantitative insights they provide. Examples are drawn from across the single-molecule literature, ranging from ultrahigh vacuum scanning tunneling microscopy studies of adsorbate diffusion on surfaces to fluorescence studies of protein conformational changes in solution.
Figures




















Similar articles
-
The scanning tunnelling microscope as an operative tool: doing physics and chemistry with single atoms and molecules.Philos Trans A Math Phys Eng Sci. 2004 Jun 15;362(1819):1207-16. doi: 10.1098/rsta.2004.1373. Philos Trans A Math Phys Eng Sci. 2004. PMID: 15306472 Review.
-
Light emission induced by tunneling electrons from surface nanostructures observed by novel conductive and transparent probes.Microsc Res Tech. 2004 Aug;64(5-6):403-14. doi: 10.1002/jemt.20099. Microsc Res Tech. 2004. PMID: 15549699
-
Extracting physics of life at the molecular level: A review of single-molecule data analyses.Phys Life Rev. 2015 Jun;13:107-37. doi: 10.1016/j.plrev.2015.01.017. Epub 2015 Jan 14. Phys Life Rev. 2015. PMID: 25660417 Review.
-
BioImaging: Contributions from biology, physics and informatics.J Biotechnol. 2010 Sep 15;149(4):227-8. doi: 10.1016/j.jbiotec.2010.08.015. J Biotechnol. 2010. PMID: 20863939 No abstract available.
-
Metrology in physics, chemistry, and biology: differing perceptions.Biol Trace Elem Res. 2007 Apr;116(1):1-4. doi: 10.1007/BF02685914. Biol Trace Elem Res. 2007. PMID: 17634623
Cited by
-
Single-molecule lysozyme dynamics monitored by an electronic circuit.Science. 2012 Jan 20;335(6066):319-24. doi: 10.1126/science.1214824. Science. 2012. PMID: 22267809 Free PMC article.
-
An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes.Biotechnol Biotechnol Equip. 2015 Mar 4;29(2):205-220. doi: 10.1080/13102818.2015.1008192. Epub 2015 Feb 17. Biotechnol Biotechnol Equip. 2015. PMID: 26019635 Free PMC article.
-
A Versatile DNA Origami-Based Plasmonic Nanoantenna for Label-Free Single-Molecule Surface-Enhanced Raman Spectroscopy.ACS Nano. 2021 Apr 27;15(4):7065-7077. doi: 10.1021/acsnano.1c00188. Epub 2021 Apr 19. ACS Nano. 2021. PMID: 33872513 Free PMC article.
-
Biophysics in drug discovery: impact, challenges and opportunities.Nat Rev Drug Discov. 2016 Oct;15(10):679-98. doi: 10.1038/nrd.2016.123. Epub 2016 Aug 12. Nat Rev Drug Discov. 2016. PMID: 27516170 Review.
-
Electrostatic catalysis of a Diels-Alder reaction.Nature. 2016 Mar 3;531(7592):88-91. doi: 10.1038/nature16989. Nature. 2016. PMID: 26935697
References
-
- Müller E. W. Das Feldionenmikroskop. Z. Angew. Phys. 1951, 131, 136–142.
-
- Melmed A. J.; Müller E. W. Study of Molecular Patterns in the Field Emission Microscope. J. Chem. Phys. 1958, 29, 1037–1041.
-
- Moerner W. E.; Kador L. Optical-Detection and Spectroscopy of Single Molecules in a Solid. Phys. Rev. Lett. 1989, 62, 2535–2538. - PubMed
-
- Binnig G.; Rohrer H. Scanning Tunneling Microscopy. Helv. Phys. Acta 1982, 55, 726–735.
-
- Smith S. B.; Finzi L.; Bustamante C. Direct Mechanical Measurements of the Elasticity of Single DNA-Molecules by Using Magnetic Beads. Science 1992, 258, 1122–1126. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

