Function of the CysD domain of the gel-forming MUC2 mucin
- PMID: 21338337
- PMCID: PMC3195396
- DOI: 10.1042/BJ20102066
Function of the CysD domain of the gel-forming MUC2 mucin
Abstract
The colonic human MUC2 mucin forms a polymeric gel by covalent disulfide bonds in its N- and C-termini. The middle part of MUC2 is largely composed of two highly O-glycosylated mucin domains that are interrupted by a CysD domain of unknown function. We studied its function as recombinant proteins fused to a removable immunoglobulin Fc domain. Analysis of affinity-purified fusion proteins by native gel electrophoresis and gel filtration showed that they formed oligomeric complexes. Analysis of the individual isolated CysD parts showed that they formed dimers both when flanked by two MUC2 tandem repeats and without these. Cleavages of the two non-reduced CysD fusion proteins and analysis by MS revealed the localization of all five CysD disulfide bonds and that the predicted C-mannosylated site was not glycosylated. All disulfide bonds were within individual peptides showing that the domain was stabilized by intramolecular disulfide bonds and that CysD dimers were of non-covalent nature. These observations suggest that CysD domains act as non-covalent cross-links in the MUC2 gel, thereby determining the pore sizes of the mucus.
Figures
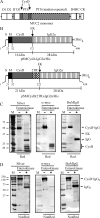

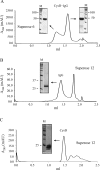




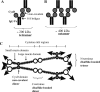
References
-
- Perez-Vilar J., Hill R. L. The structure and assembly of secreted mucins. J. Biol. Chem. 1999;274:31751–31754. - PubMed
-
- Thornton D. J., Rousseau K., McGuckin M. A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008;70:459–486. - PubMed
-
- Gum J. R., Hicks J. W., Toribara N. W., Siddiki B., Kim Y. S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 1994;269:2440–2446. - PubMed
-
- Li D., Gallup M., Fan N., Szymkowski D. E., Basbaum C. B. Cloning of the amino-terminal and 5′-flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts. J. Biol. Chem. 1998;273:6812–6820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

