Maternal characteristics during pregnancy and risk factors for positive HIV RNA at delivery: a single-cohort observational study (Brescia, Northern Italy)
- PMID: 21338498
- PMCID: PMC3058020
- DOI: 10.1186/1471-2458-11-124
Maternal characteristics during pregnancy and risk factors for positive HIV RNA at delivery: a single-cohort observational study (Brescia, Northern Italy)
Abstract
Background: Detectable HIV RNA in mothers at delivery is an important risk factor for HIV transmission to newborns. Our hypothesis was that, in migrant women, the risk of detectable HIV RNA at delivery is greater owing to late HIV diagnosis. Therefore, we examined pregnant women by regional provenance and measured variables that could be associated with detectable HIV RNA at delivery.
Methods: A observational retrospective study was conducted from January 1999 to May 2008. Univariate and multivariable regression analyses (generalized linear models) were used, with detectable HIV RNA at delivery as dependent variable.
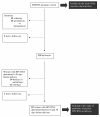
Results: The overall population comprised 154 women (46.8% migrants). Presentation was later in migrant women than Italians, as assessed by CD4-T-cell count at first contact (mean 417/mm³ versus 545/mm³, respectively; p = 0.003). Likewise, HIV diagnosis was made before pregnancy and HAART was already prescribed at the time of pregnancy in more Italians (91% and 75%, respectively) than migrants (61% and 42.8%, respectively). A subgroup of women with available HIV RNA close to term (i.e., ≤30 days before labour) was studied for risk factors of detectable HIV RNA (≥50 copies/ml) at delivery. Among 93 women, 25 (26.9%) had detectable HIV RNA. A trend toward an association between non-Italian nationality and detectable HIV RNA at delivery was demonstrated by univariate analysis (relative risk, RR = 1.86; p = 0.099). However, by multivariable regression analysis, the following factors appeared to be more important: lack of stable (i.e., ≥14 days) antiretroviral therapy at the time of HIV RNA testing (RR = 4.3; p < 0.0001), and higher CD4+ T-cell count at pregnancy (per 50/mm³, RR = 0.94; p = 0.038).
Conclusions: These results reinforce the importance of extensive screening for HIV infection, earlier initiation of antiretroviral therapy and stricter monitoring of pregnant women to reduce the risk of detectable HIV RNA at delivery. Public health interventions should be particularly targeted to migrant women who are frequently unaware of their HIV status at the time of pregnancy.
Figures
References
-
- UNAIDS. AIDS Epidemic Update. 2009. http://www.unaids.org/en/media/unaids/contentassets/dataimport/pub/repor... last accessed on 30th September 2010.
-
- CDC. Progress toward elimination of perinatal HIV infection-Michigan 1993-2000. MMWR Morb Mortal Wkly Rep. 2002;51:93–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials