Heterotaxin: a TGF-β signaling inhibitor identified in a multi-phenotype profiling screen in Xenopus embryos
- PMID: 21338922
- PMCID: PMC3050558
- DOI: 10.1016/j.chembiol.2010.12.008
Heterotaxin: a TGF-β signaling inhibitor identified in a multi-phenotype profiling screen in Xenopus embryos
Abstract
Disruptions of anatomical left-right asymmetry result in life-threatening heterotaxic birth defects in vital organs. We performed a small molecule screen for left-right asymmetry phenotypes in Xenopus embryos and discovered a pyridine analog, heterotaxin, which disrupts both cardiovascular and digestive organ laterality and inhibits TGF-β-dependent left-right asymmetric gene expression. Heterotaxin analogs also perturb vascular development, melanogenesis, cell migration, and adhesion, and indirectly inhibit the phosphorylation of an intracellular mediator of TGF-β signaling. This combined phenotypic profile identifies these compounds as a class of TGF-β signaling inhibitors. Notably, heterotaxin analogs also possess highly desirable antitumor properties, inhibiting epithelial-mesenchymal transition, angiogenesis, and tumor cell proliferation in mammalian systems. Our results suggest that assessing multiple organ, tissue, cellular, and molecular parameters in a whole organism context is a valuable strategy for identifying the mechanism of action of bioactive compounds.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
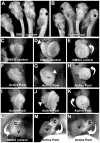
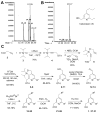
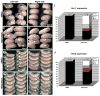

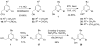

References
-
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. - PubMed
-
- Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5:575–585. - PubMed
-
- Bertolino P, Deckers M, Lebrin F, ten Dijke P. Transforming growth factor-beta signal transduction in angiogenesis and vascular disorders. Chest. 2005;128:585S–590S. - PubMed
-
- Bols M, Skrydstrup T. Silicon-Tethered Reactions. Chemical Reviews. 1995;95:1253–1277.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

